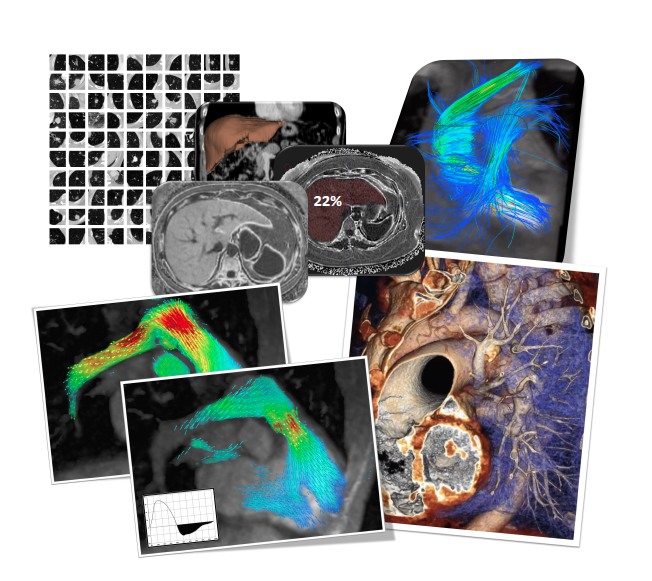
A team of researchers at the University of Washington announced they developed a new automated system for producing zirconium-89, a diagnostic radionuclide used for cancer tumor imaging.
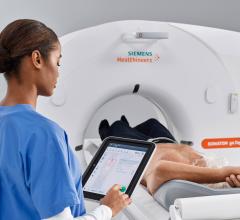
Siemens Healthineers will introduce the Somatom go.Top Cardiovascular Edition, a new version of its established computed tomography (CT) system, at the 68th Annual Scientific Session and Expo of the American College of Cardiology (ACC), March 16-18 in New Orleans. The Somatom Go.Top Cardiovascular Edition is designed to deliver personalized patient dose control in all types of routine cardiovascular imaging. The cost-effective, 128-slice scanner offers outpatient cardiology offices as well as hospitals access to not just coronary CT angiography (CCTA), but also advanced tests, including the HeartFlow FFRCT (fractional flow reserve computed tomography) Analysis.
The number of new particle therapy rooms ordered worldwide dropped by almost 20 percent in 2018, according to a new report from MEDraysintell, continuing a trend dating back to mid-2016. The report says this trend may change if particle therapy vendors are able to build a strong order book in 2019 and 2020.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Esaote announced the launch of MyLab X8, a high-performance, versatile ultrasound platform to support hospitals and clinics facing today’s challenges, at the 2019 European Congress of Radiology (ECR), Feb. 27-March 3 in Vienna, Austria.
An imaging procedure commonly performed before starting cancer treatment can provide valuable clues about a patient's risk for heart problems in the months and years after treatment. However, this information is not always reported and is rarely acted upon in current practice, according to research being presented at the American College of Cardiology's 68th Annual Scientific Session, March 16-18 in New Orleans.
At RSNA 2018, iCad showed how its ProFound AI for digital breast tomosynthesis technology might help in the ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Genomic medicine company Lucence Diagnostics announced a new project to develop artificial intelligence (AI) algorithms for improving diagnosis and treatment of liver cancer. The goal is to combine the imaging and molecular data from liver cancer patients into smarter software tools that help physicians make better treatment decisions.Genomic medicine company Lucence Diagnostics announced a new project to develop artificial intelligence (AI) algorithms for improving diagnosis and treatment of liver cancer. The goal is to combine the imaging and molecular data from liver cancer patients into smarter software tools that help physicians make better treatment decisions.
March 12, 2019 — A simple magnetic resonance imaging (MRI) method that measures iron content can provide a more ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Computed tomography (CT) and magnetic resonance imaging (MRI) scans are chock full of information that might be used to ...
Artificial intelligence (AI) startup company Paige.AI has been granted Breakthrough Device designation by the U.S. Food and Drug Administration (FDA) for its AI designed to enhance the clinical diagnosis and treatment of cancer. It is the first such designation for AI in cancer diagnosis publicly announced by any company, according to Paige.AI.
Anderson Regional Cancer Center in Meridian, Miss., has treated its first patient using the combination of RaySearch's treatment planning system (TPS) RayStation and Accuray's TomoTherapy Treatment Delivery System. This is the first-ever treatment in the U.S. using the two technologies in combination.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
March 8, 2019 — Imaging Technology News (ITN) was named a finalist in multiple categories across print, web and other ...
Mike Ciancio, imaging systems administrator at CarolinaEast Health System in North Carolina, explains how newer ...
Karl Poterack, M.D., medical director, applied clinical informatics, Mayo Clinic, explains the role wearable devices ...
A new dosimetry monitoring service from Thermo Fisher Scientific enables medical and imaging facilities, dental offices, veterinary clinics, nuclear power plants, laboratories and other facilities with radiation safety requirements to streamline management of their safety programs.
National and international ultrasound societies are urging the U.S. Food and Drug Administration to remove the “black box” from ultrasound contrast agent (UCA) labels.
Hologic Inc. announced the granting of a CE Mark to the LOCalizer wireless radio frequency identification (RFID) breast lesion localization system. This system is designed for precise and easy marking and targeting of lesions for breast-conserving surgery guidance.
Carestream Health has signed an agreement to sell its healthcare information systems (HCIS) business to Philips ...


 March 14, 2019
March 14, 2019