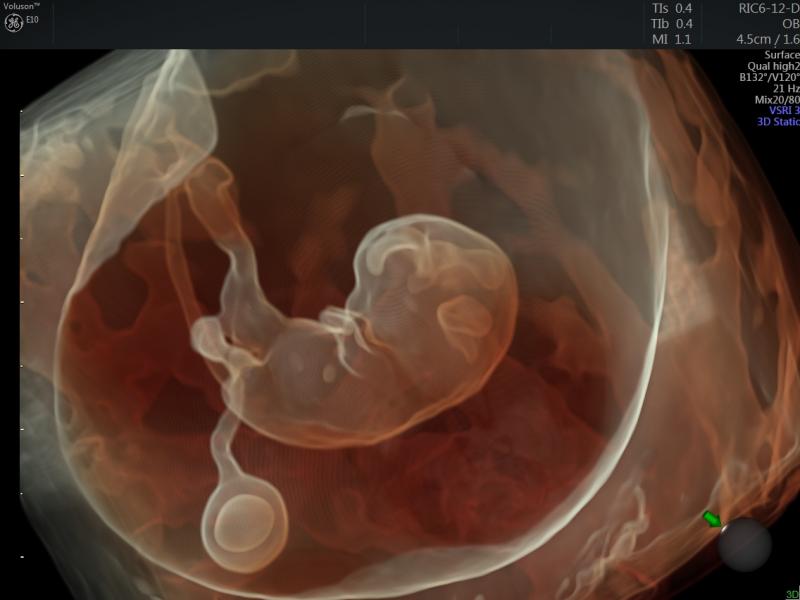
Below is a collection of prenatal ultrasound pictures from the Imaging Technology News (ITN) archive. Use the arrows to ...
May 8, 2018 — In a first, an international research team from the Department of Biopsychology at Ruhr-Universität Bochum ...
NeuroLogica, a subsidiary of Samsung Electronics Co. Ltd., announced the Samsung RS85 ultrasound system has received U.S. Food and Drug Administration (FDA) 510(k) clearance. The RS85 is the latest expansion of Samsung’s ultrasound portfolio.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
A new data analysis of the Northwestern Medicine Mobile Stroke Unit (MSU) found the specialized ambulance provided life-saving treatment 30 minutes faster than traditional transport in its first year of operation. The analysis found, on average, the MSU delivered the clot-busting drug tPA to ischemic stroke patients 52 minutes after 9-1-1 dispatch, compared to an average of 82 minutes for patients transported via ambulance.
The teenager's chest was caved in. The underlying congenital flaw had gotten so bad that the sternum was almost pressing against the spine. Surgery at Phoenix Children's Hospital (PCH) in Phoenix saved the boy's life. A three-dimensional volume rendering of his chest paved the way. With it, the surgical team at PCH planned the operation.
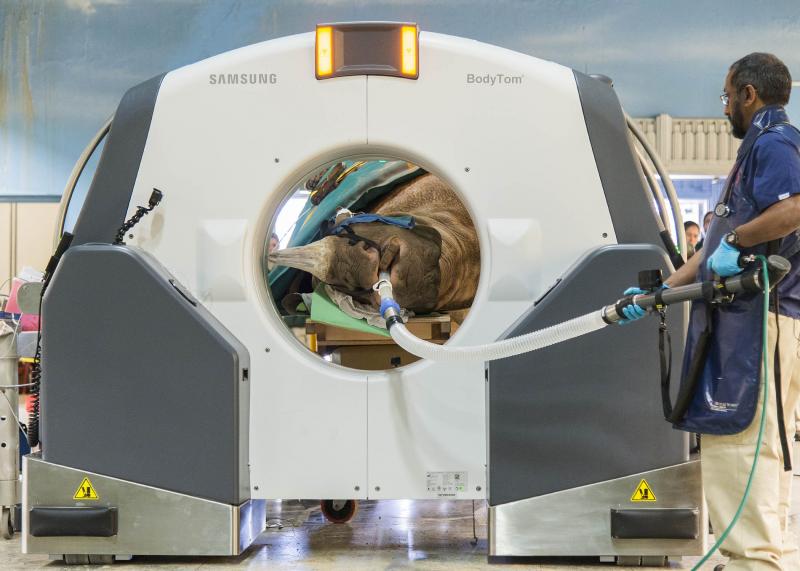
On April 19, 2018, Layla, a 7½ -year-old, 2,300-pound eastern black rhinoceros who lives at Brookfield Zoo, underwent what is believed to be the first computed tomography (CT) scan ever performed on this species. The scan was necessary to help Chicago Zoological Society (CZS) veterinarians determine the next steps for treating a known obstruction in Layla’s nasal passageway.
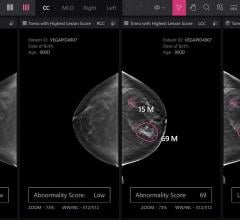
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
May 3, 2018 — The U.S. Food and Drug Administration (FDA) recently announced the availability of a draft guidance for ...
With the support of the Austrian Science Fund FWF, researchers from Vienna have developed methods to improve functional magnetic resonance imaging (MRI) with the new generation of highly sensitive 7 Tesla (7T) scanners. These devices can create precise maps of the brain before surgery to help surgeons avoid damaging vital areas.
A 360 degree view of the newest cath lab at Northwestern Medicine Central DuPage Hospital in Winfield, Ill., located in ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
This is a 360 photo view of a recent Carestream DRX Excel Plus radiographic fluoroscopy (R/F) room installation at ...
We are a nation in search of simple solutions. We want silver bullets to slay whatever ails us. Value-based medicine is ...
The global breast imaging market is expected to reach $7.3 billion by 2024, according to a new report by Grand View ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Clinical research has revealed men and women often have different presentations for cardiovascular disease (CVD). This ...
As a radiologist in today’s evolving global healthcare landscape, I’m grateful for the innovations in medical technology ...
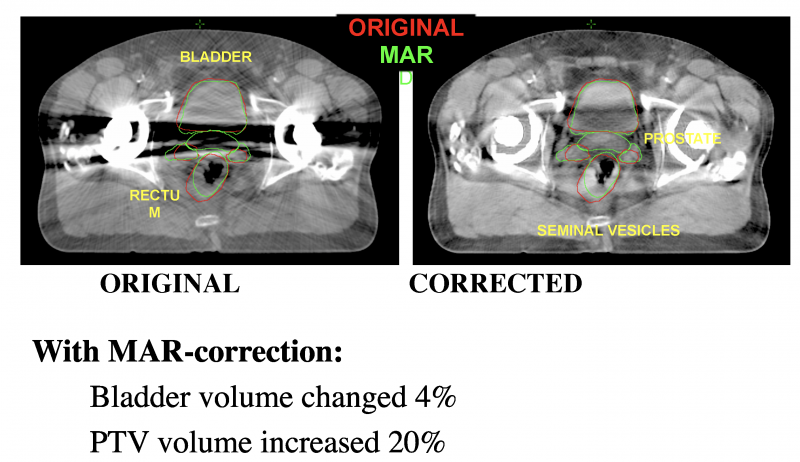
Computed tomography (CT) has long been the standard of care for imaging to plan radiation therapy (RT) treatments.
May 3, 2018 — BWX Technologies Inc. signed a definitive agreement to acquire Sotera Health’s Nordion medical isotope ...
Magnetic resonance imaging (MRI) for cardiac assessment provides a radiation-free alternative to other commonly used modalities like computed tomography (CT) and single photon emission computed tomography (SPECT). Cardiac MRI (CMR) offers greater contrast and image clarity than CT, does not require use of a contrast agent and allows radiation-free perfusion imaging. The technology does, however, present challenges unseen with other modalities. It remains the most expensive of all imaging exams, largely thanks to the length and complications of the scan itself. Furthermore, the use of magnets has traditionally made MRI inaccessible for patients with implanted medical devices.
The concept of picture archiving and communication systems (PACS) has been part of radiology since the early 1970s. The technology has provided a way to hold and view large volumes of medical imaging data as radiology has transitioned away from analog image acquisition. In recent years, however, the rise of enterprise imaging has changed the role of PACS, and providers are looking for new, modern solutions, according to a 2017 report from independent research firm KLAS. Surveying thousands of healthcare professionals, the report found that performance from historically popular PACS vendors has declined, and newer market players have begun to set themselves apart.
Consolidation of health information is key to providing a comprehensive patient record —not only for visibility across the continuum of care, but also as the record set for value-based care. The advent of the electronic medical record (EMR) has pushed organizations into consolidating and collecting as much clinical information as possible within a single contextual data library. As this data feeds into the clinical data library, it can be visualized as a component of the patient record within the EMR. Traditionally, this contextual data alone could be considered an organization’s health information library. However, clinical contextual data by itself represents only a partial view of the patient record.
As healthcare systems continue to expand through consolidation and the amount of data generated by patients grows ...

 May 07, 2018
May 07, 2018