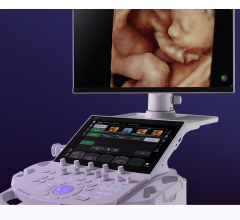
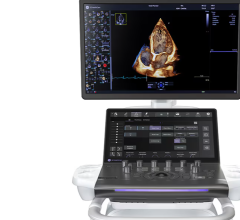
May 7, 2018 — NeuroLogica, a subsidiary of Samsung Electronics Co. Ltd., announced the Samsung RS85 ultrasound system has received U.S. Food and Drug Administration (FDA) 510(k) clearance. The RS85 is the latest expansion of Samsung’s ultrasound portfolio.
The RS85 features an array of improvements, including:
- MV-Flow: Allows for visualization of slow-flow micro vascularized structures, which can be difficult to assess with conventional power Doppler ultrasound. It also provides clinicians an additional way to check lesions for indications of cancer or inflammation;
- S-Shearwave Imaging: Provides new indicators for clinical diagnosis by quantifying the elasticity of tissue or lesions via shearwave elastography, which may help increase the accuracy of diagnosis for breast and liver diseases;
- CEUS+: Diagnoses and characterizes lesions in the liver using a contrast agent in adult and pediatric populations; and
- S-Fusion: Enables simultaneous localization of a lesion using real-time ultrasound in conjunction with other volumetric imaging modalities such as a magnetic resonance image (MRI) or a computed tomography (CT) scan, while expanding its capabilities to the prostate gland for precise, targeted biopsy guidance.
The RS85 was also developed to help decrease user-fatigue and repetitive motions with an enhanced monitor arm, and increased range of motion and tilt. These improvements allow the user to position the monitor for optimal viewing and control. Additionally, multi-step actions have been combined into a single step to help reduce keystrokes and repetitive user interface interactions.
For more information: www.neurologica.com


 February 05, 2026
February 05, 2026