Accelerator physicists are natural-born problem solvers, finding ever more powerful ways to generate and steer particle beams for research into the mysteries of physics, materials and matter. And from the very beginning, this field born at the dawn of the atomic age has actively sought ways to apply advanced technologies to tackle more practical problems. At the top of the list—even in those early days— was taking aim at cancer.
Decompressing after RSNA 2013, I took a moment to watch “Falling Down” with Michael Douglas. I always read the credits at the end of movies, mostly for the bit players. The main characters everyone knows — the Douglases and the Duvalls. I feel like the bit characters deserve some attention. There, five down from the top and two above Mr. De Fens’ mother, was a credit that shook me — Tuesday Weld as Mrs. Prendergast.
Regulatory and legislative changes have always resulted in sleepless nights for stakeholders in the radiology industry due to the need to understand implications, interpretations and timeline facets that influence usage, workflow, payment, adoption, procurement and, eventually, choice. Legislation involves almost everyone in the value chain from original equipment manufacturer (OEM) research and development folks, product management, marketers, OEM sales and application specialists, hospital and radiology administrators, radiologists, radiographers, biomedical engineers and even finance staff linked to a radiology department.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
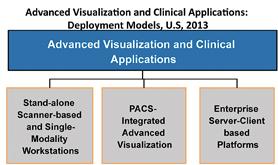
If the exhibit floor of the 99th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA) had one standout modality this year, it was, without question, the advances with premium computed tomography (CT). Technological and clinical advances in magnetic resonance (MR) and molecular imaging were less impressive but were no less significant. Naturally, these developments in every advanced imaging modality are turning eyes to advanced visualization (AV) and clinical applications. AV is not just a core step in the interpretation workflow of each of these modalities. More than that, it is the set of solutions that will allow the realization of the promises of these imaging advances — the ones that will “make sense” of these upgraded imaging capabilities by using innovative ways to interrogate their improved image depth and visualize their enhanced image quality.
The introduction of iPhones and iPads revolutionized the way people communicate and made transmitting large amounts of information more convenient and instantaneous. With the expansion into different types of smartphones and tablets — Android- and Windows-based — society has grown increasingly dependent on these devices to transmit and receive information, and this dependence has greatly impacted healthcare. Morris Panner, CEO of DICOM Grid, said that, “For the new generation of physicians, using mobile technology is as common for many of them as a stethoscope. They walk into medical school with one of these devices and expect to receive information on it.”
The Cancer Center at Banner Health McKee Medical Center in Loveland, Colo., boasts an array of state-of-the-art technologies and well-qualified staff, but at the core of its mission is the northern Colorado community it serves. “The community is significant, as many local funds were raised for the cancer center, which definitely makes it a community center,” said McKee Cancer Center Director Cindy McBlair, MHA, (R)(T).
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Screening mammography is the key to reducing mortality from breast cancer, but women without insurance or the ability to pay do not have easy access to the life-saving exam. That’s something SouthCoast Imaging is working to change. The private imaging practice has been donating 2D mammograms to uninsured women in its community for years, and recently introduced an innovative fundraising program to make 3D mammograms available to more women. SouthCoast donates one 3D mammogram to a woman without healthcare for every 10 screening 3D mammograms they perform.
Throughout the years, imaging technologies have continued to improve for the screening and detection of cancer in the breast. While traditional mammography, ranging from film-screen to full-field digital mammography (FFDM) remains the gold standard for routine screening of women over 40 (or women over 50, per the U.S. Preventive Services Task Force guidelines that were issued in 2009), enhanced forms of mammography, as well as uses for the combination of mammography with other imaging modalities, are emerging.
Criticism and confusion notwithstanding regarding the appropriateness of X-ray mammography as the gold standard in breast screening, digital mammography will continue to provide an important cog in the wheel of breast care pathways. The United States, some may say, is a slowing market for the digital mammography imaging modality due to a gradual decrease in the number of breast imaging facilities, reduction in replacement rates of mammography units and the possible linkage of reduced procedure numbers to the 2009 U.S. Preventive Services Task Force (USPSTF) recommendations.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Better management of X-ray radiation doses starts with recording and tracking each exposure patients receive. Dose tracking has come to the forefront of medicine in recent years with the realization that medical imaging has doubled the public’s exposure to ionizing radiation since the 1980s, largely due to the rapid expansion of computed tomography (CT) and minimally invasive angiography procedures.
A big trend in healthcare IT at Healthcare Information and Management Systems Society (HIMSS) 2014 annual meeting was ...
Even with the best intentions, many women put off their annual mammogram. It’s one more appointment to schedule, one more trip to the imaging center or hospital; and, with so many demands on their time, many women don’t make the time. One company is working to increase compliance by helping physician practices provide their patients with in-office mammograms.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
This industry has watched a trend in medical technology grow from a small ripple a couple of years ago into a potential tidal wave in 2014 due to growing concern over patient radiation dose levels from medical imaging. Use of radiation dose monitoring software came to the forefront when California, followed by Texas, created laws requiring medical facilities to record the amount of exposure patients receive from tests such as computed tomography (CT) and magnetic resonance imaging (MRI).
By Dave Fornell, editor With healthcare reform’s focus on information technology as the primary vehicle for change, there has been a massive increased interest in this area. Health IT software has taken center stage as healthcare facilities look to implement new Stage 2 Meaningful Use requirements. This year the Healthcare Information and Management Systems Society (HIMSS) annual meeting, held Feb. 23-27 in Orlando, Fla., drew a record crowd of 38,828 attendees and a record number of 1,233 exhibiting vendors.
Elekta demonstrated new features in its Mosaiq oncology information system (OIS) at HIMSS14. Elekta highlighted functionality optimized for today's rapidly consolidating oncology care delivery systems.
The American College of Radiology applauded steps to reign in medical imaging and radiation oncology self-referral included in President Obama’s Fiscal Year 2015 budget. However, prior authorization for imaging services, also included the FY 2015 budget, is unnecessary and will ultimately raise costs, interfere in the doctor-patient relationship and restrict ready access to imaging care.
Vital Images Inc., a Toshiba Medical Systems Group Company, is expanding in the EMEA market with an increasing number of organizations migrating to Vital's VitreaAdvanced solution to help improve efficiency, communication and patient care. The VitreaAdvanced advanced visualization solution provides 2-D, 3-D and 4-D images for applications addressing cardiovascular, neurovascular and oncology disease states. Fueled by intelligent automation, it utilizes a clinical workflow to improve speed and simplicity of use.
The BioZorb 3-D surgical marker improved targeting for radiation therapy by enhancing visualization of the breast cancer lumpectomy cavity. This is according to a presentation at the 2014 Breast Cancer Coordinated Care (BC3) conference in Washington D.C.
The Finnish mammography provider Suomen Radiologikeskus Oy signed an agreement with the IT and medical technology company Sectra. Togethery they will provide osteoporosis assessment in conjunction with public mammography screening in all of Keskus’ nine mammography centers in Finland. Women can have their bone health examined at the same time as their regular mammogram, enabling early detection of osteoporosis and reducing the risk of future fractures.
Interoperability, mobile connectivity and technologies that drive real-time actionable information at the point of care will be the focus of health information technology (IT) investments in 2014. This is according to the results of a new healthcare leadership survey released by Philips Healthcare.

 March 07, 2014
March 07, 2014