Until recently, cancer patients looking for a precise form of proton radiation therapy – "pencil-beam scanning" – had to narrow their search to about a half dozen centers in the United States that offer this treatment on a limited basis.
Guerbet, a contrast agent specialist for medical imaging, received U.S. Food and Drug Administration (FDA) approval for a manufacturing plant for Lipiodol (ethiodized oil) Injection, in Montreal, Canada.
VitreaView, a universal viewer developed by Vital Images Inc. was featured in a live case study. Shafiq Rab, M.D., vice president and chief information officer at Hackensack University Medical Center, presented during the Interoperability Showcase, Feb. 25 at HIMSS 2014 in Orlando, Fla.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Viztek, a provider of digital software and hardware diagnostic imaging solutions, announced 45 percent year-over-year revenue growth from the 2011-2013 calendar years. The company experienced 20 percent growth in digital radiography (DR), and 15 percent growth in software. The PACS replacement market was particularly strong for the company, with 75 replacements in 2013. In the same year, Viztek also saw a significant jump in its customer base of hospitals with more than 200 beds.
Results of the 2013 HIMSS Security Survey show progress toward hardened security and use of analytics, but more work is needed to mitigate insider threat, such as inappropriate access of data by employees. Federal initiatives such as OCR audits, meaningful use (MU) and the HIPAA Omnibus Rule encourage healthcare organizations to increase the budgets and resources dedicated to securing patient health data. However, over the past year, 19 percent of respondents reported a security breach. Additionally, 12 percent of organizations have had at least one known case of medical identity theft reported by a patient.
The latest Soapware includes an order manager and continued progress toward 2014 meaningful use (MU). These features aim to deliver clinical value with maximum ease of use and also to meet MU 2014 requirements. Further features will be gradually added in future updates to continue to allow for a smooth transition and ample training time of MU 2014.
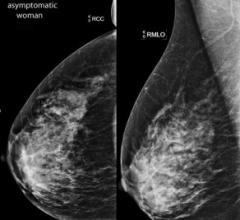
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Philips partnered with SonoCiné Inc., a U.S. research, development and manufacturing company, to provide automated whole breast ultrasound (AWBUS) imaging for the Philips EPIQ and iU22 ultrasound systems.
The market for electronic health and medical records (EMRs) is set to experience rapid growth over the coming years, with EMR peer group value estimated to climb from approximately $10.6 billion in 2012 to $17 billion by 2017, at a Compound Annual Growth Rate (CAGR) of 9.8 percent, according to research and consulting firm GlobalData.
Senates in Missouri (SB 639) and the state of Washington (SB 6050) have both advanced Breast Density Inform bills. The bills are now headed to their respective Houses of Representatives. Rhode Island has also introduced a Breast Density Inform bill (H7341).
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
HIT Application Solutions announced a partnership with Merge Healthcare Inc. to integrate its Notifi platform with Merge RIS, a Meaningful Use (MU) outpatient radiology information system. The solution, to be marketed as Merge Notifi, will initially automate appointment reminders and make patient results available through messages for Merge customers. Merge Notifi is a HIPPA compliant service.
Claron Technology will debut at HIMSS 2014 enhancements for a streamlined image and video upload for both mobile devices and desktop PCs. This will add functionality to its family of Nil universal, zero-footprint viewers. The technology requires a browser only, not installation of an app or engine. The technology supports mobile devices and allows zero-footprint upload of both digital imaging and communications in medicine (DICOM) and non-DICOM data from notebook and desktop computers.
The U.S. Food and Drug Administration (FDA) cleared Carestream Health’s Vue Motion image viewer for clinical viewing and reading of medical X-ray exams using iPhone 4s, iPad 2, Galaxy Note and Galaxy S III mobile devices.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Fovia, Inc. and Visual Medica announced a collaboration to deliver innovative, cost-effective advanced visualization to the Latin American market.
At HIMSS 2014, Sectra exhibited updates in PACS and imaging workflow. Adar Palis, executive vice president and chief operating officer of Harrison Medical Center, will present Northwest ImageShare. The collaboration between competitors that enables economies-of-scale pricing, system standardization for physicians and improved patient care was presented at HIMSS, Feb. 27.
Merge Healthcare Inc.’s radiology solutions have been certified for Meaningful Use (MU). Merge PACS, iConnect Access and Merge RIS are all now Office of the National Coordinator Authorized Certification Body (ONC-ACB) certified under the 2014 criteria.?
Carestream is sponsoring an executive panel discussion at the HIMSS 2014 meeting, Feb. 25, from 3:30 to 4:30 p.m. in Room 202C at the Orlando Convention Center. The title is “Executive Perspective: How to Achieve Efficient Enterprise Data Management” and the session is open to all HIMSS attendees. Jennifer Horowitz, Senior Director of Research for HIMSS Analytics, will be the moderator.
The American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO) published a consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. The guideline document represents an intensive collaboration among experts in the radiation oncology and surgical oncology fields, led by Meena S. Moran, M.D., associate professor of the department of therapeutic radiology at Yale School of Medicine in New Haven, Conn., on behalf of ASTRO, and Monica Morrow, M.D., SSO immediate past president, breast cancer surgeon and chief of breast surgery at Memorial Sloan-Kettering Cancer Center in New York, co-chairs of the Margin Consensus Panel (MCP). In addition to determining the ideal margin width that minimizes the risk of ipsilateral breast tumor recurrence (IBTR), the guideline outlines an evidence-based surgical treatment path that could reduce unnecessary surgery for patients.
Wolters Kluwer Health, a global provider of information for healthcare professionals and students, will make available at HIMSS13 Annual Conference & Exhibition the UpToDate. Anywhere. The product enables healthcare enterprises to improve patient care by equipping clinicians with anytime/anywhere access to comprehensive, evidence-based, clinical decision support through the UpToDate mobile app.
The AT Group LLC, Booth 7384 within the First Time Exhibitor Pavilion, is featuring its portfolio companies, including MatrixView and its PACStream Solution at HIMSS.
By Melinda Taschetta-Millane, editorial director With the theme of “Innovation. Impact. Outcomes. Onward,” HIMSS14, the annual conference and exhibition of the Healthcare Information and Management Systems Society, will hold its annual event February 23-27, 2014, at the Orange County Convention Center in Orlando.


 February 20, 2014
February 20, 2014