Spurred by provisions of the Affordable Care Act (ACA) and other legislation, usage of breast cancer screening mammography and related follow-on diagnostic testing is expected to increase in the coming years. Yet there is wide variation in the utilization and sequence of follow-on testing on a patient-by-patient basis, including a high rate of “false positive” for biopsies. This suggests better methods for selecting suspicious lesions could reduce the number of unnecessary biopsies.
Research shows that ultra-high-field magnetic resonance imaging (MRI) provides detailed views of a brain area implicated in Parkinson’s disease, possibly leading to earlier detection of the condition. The results of this research are published online in the journal Radiology.
Patients who received daily humidification of the mouth and throat region beginning from day one of radiation therapy treatment spent nearly 50 percent fewer days in the hospital to manage their side effects, according to research presented today at the 2014 Multidisciplinary Head and Neck Cancer Symposium.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Advanced Accelerator Applications, an international specialist in molecular nuclear medicine (MNM), announced it has entered into an agreement to acquire 100 percent of the shares of Imaging Equipment Ltd., a privately held U.K. distributor of nuclear medicine products and technologies.
Research by Beaumont Health System radiation oncologists and neurosurgeons found that symptoms of trigeminal neuralgia (TN) were reduced in those treated with Gamma Knife stereotactic radiosurgery. The results were published in the February issue of the journal Clinical Neurology and Neurosurgery.
We all use computer monitors for our jobs on a daily basis, but most are not configured for an ideal ergonomic workstation. Here are a few suggestions to achieve the best results from ergonomic workstations vendor AFC Industries Inc.
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
At HIMSS14, Microsoft Corp. showcased a range of health organizations, including Epic and AirStrip, that have improved care team collaboration and productivity, expanded mobility and enhanced patient care through Windows 8.1 devices and apps, and Windows Embedded solutions. With the Windows 8.1 platform of clinical-grade devices, apps and services, including Microsoft Surface, mobile care teams can: communicate with each other via a variety of methods; gain access to full-function EMRs from anywhere at any time; and use U.S. Food and Drug Administration (FDA) -cleared clinician apps to deliver mobile-based care and guidance.
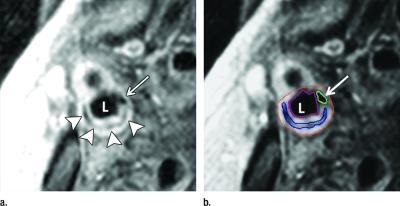
Noninvasive imaging of carotid artery plaque with magnetic resonance imaging (MRI) can accurately predict future cardiovascular events like strokes and heart attacks in people without a history of cardiovascular disease, according to a study published online in the journal Radiology.
March 5, 2014 — Researchers at the Cedars-Sinai Maxine Dunitz Neurosurgical Institute and Department of Neurosurgery have developed a unique, compact and relatively inexpensive imaging device to “light up” malignant brain tumors and other cancers.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
March 5, 2014 — vRad (Virtual Radiologic), the largest telemedicine company and radiology practice in the United States, announced its newest set of indices, the “Day in the Life of Radiology,” part of the RPC (Radiology Patient Care) Indices available for free and unrestricted use at vRad’s website.
March 5, 2014 — Esaote has launched CrystaLine, a significant evolution of its ultrasound technology that delivers new levels of accuracy, quality, versatility and value. Esaote will showcase the new technology at the 2014 annual meeting of the European Congress of Radiology in Vienna, March 6-10.
March 5, 2014 — Claron Technology announced it has been awarded CE mark for its NilRead zero-footprint viewer for the diagnostic interpretation of medical images. Coinciding with this, Claron has opened its European headquarters in Hasselt, Belgium, under the direction of Tom Tilmans, director of sales. In addition to spearheading direct sales efforts, Tilmans will recruit and manage a team of European partners and distributors.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
March 4, 2014 — Advanced Accelerator Applications (AAA), a growing international player in molecular nuclear medicine, announced they have received orphan drug designation status for their radiopharmaceutical, Gallium-68 Dotatate.
March 4, 2014 — Physicians’ Education Resource (PER) announced that “The Great Mammography Debate” has been added to the 31st annual Miami Breast Cancer Conference agenda, in light of a controversial study that suggests mammograms do not reduce the number of women who die from breast cancer. Registration is open for the conference, which will be hosted at the Fontainebleau Miami Beach, March 6-9.
National Decision Support Co. (NDSC) enhanced its ACR Select, an integration-ready, imaging decision support platform based on American College of Radiology (ACR) appropriateness criteria.
Fujifilm Medical Systems USA Inc. unveiled a patient-centric vendor neutral archive (VNA) for healthcare professionals at HIMSS 2014 in Orlando, Fla., Feb. 23-27. Synapse VNA technology will catalog and maintain the data in a patient centric model allowing a single access point for EMRs and other systems.
Eizo Inc. released two 27-inch ColorEdge models, the ColorEdge CG277 and CX271 color management monitors.
CliniComp expanded into outpatient and ancillaries with its CliniComp EHR, showcased at HIMSS 2014. The solution integrates order entry (CPOE) and order/results management, which includes order tracking. The solution allows creation of radiology specific CPOE templates based on rules defined by the facility, based on guidelines, protocols, CPT codes, reimbursement or other parameters.
DR Systems exhibited capabilities designed to increase the quality and efficiency of patient services at HIMSS 2014. Also on display was Health Companion, a patient portal for secure messaging and patient engagement.
Aycan and Chimaera GmbH received U.S. Food and Drug Administration (FDA) 510(k) clearance of Chimaera FusionSync, a software tool for more efficient handling and diagnosis of multi-modal and follow-up medical data. Available with Aycan workstation OsiriX Pro, Chimaera FusionSync was launched in Europe in 2013 with CE marking.

 March 06, 2014
March 06, 2014