Researchers have validated a first-of-its-kind machine learning–based model to evaluate immunohistochemical (IHC) characteristics in patients with suspected thyroid nodules, according to an ahead-of-print article published in the December issue of the American Journal of Roentgenology (AJR). The research team achieved “excellent performance” for individualized noninvasive prediction of the presence of cytokeratin 19, galectin 3 and thyroperoxidase based upon computed tomography (CT) images.
Researchers in South Korea have found that patients with family and personal history of allergic reactions to contrast media are at risk for future reactions, according to a large study published in the journal Radiology.1 Allergic reactions to commonly used computed tomography (CT) contrast media may be prevented by premedicating patients with antihistamines and using a different type of contrast agent.
Philips will showcase its latest cardiac care innovations at the European Society of Cardiology (ESC) Congress 2019, Aug. 31–Sept. 4 in Paris, France. At the congress, Philips is showcasing Release 5.0 of its Epiq CVx cardiology platform for the first time in Europe. The platform includes automated applications for 2-D assessment of the heart, as well as robust 3-D right ventricle volume and ejection fraction measurements, making accurate exams faster and easier to conduct. Philips also announced that it is collaborating with digital health company LindaCare to combine the latter’s OnePulse cloud-based solution for the remote monitoring of patients with cardiac implantable electronic devices (CIEDs) with the Philips IntelliSpace Cardiovascular informatics platform.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Civco will display its Universal Couchtop ProForm Head & Neck Solution at the 2019 American Society for Radiation Oncology (ASTRO) annual meeting, Sept. 15-18 in Chicago.
August 30, 2019 — The CIRS Ultrasound Quality Assurance Portal helps users of CIRS general-purpose ultrasound phantoms ...
In a surprising move, the National Institute for Radioelements (IRE) has applied for a new license to export highly ...
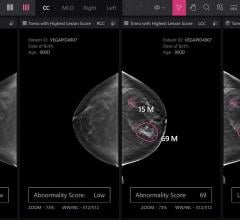
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
The clinical needs of those who live in rural America and small communities are both simple and complex. A physician may ...
Beginning with the 2019 meeting, the American Society for Radiation Oncology (ASTRO) will begin transforming its annual ...
Use of the Internet of Things (IoT) is booming, with IHS Markit forecasting there will be 73 billion connected devices in use around the world by 2025. IoT technology has moved beyond speakers and smart fridges and is increasingly being utilized for critical applications across the healthcare industry like insulin delivery devices, connected inhalers and even cancer treatments.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
A notable increase in the prevalence of chronic diseases has led to a surge in sales of high-end diagnostic machines, including magnetic resonance imaging (MRI) scanners, which in turn is fostering demand for MRI-safe pulse oximeters, according to a new market report.
Delaware Imaging Network (DIN), Delaware’s largest network of outpatient medical imaging centers, has added NeuroQuant magnetic resonance imaging (MRI) software, to brain imaging studies at all its centers that offer MRI services. NeuroQuant is a U.S. Food and Drug Administration (FDA)-cleared software used in the assessment of neurological conditions.
The University of Alabama at Birmingham, in conjunction with researchers at the University of Wisconsin and Argonne National Laboratory in Illinois, have received a Department of Energy grant to solve a production roadblock for the radioactive isotopes 43Sc and 47Sc. These radioactive isotopes of the metallic element scandium (Sc) appear to be ideal for visualizing and then destroying solid tumors. A barrier, however, blocks their use — the inability to rapidly produce and purify the isotopes in useful amounts. 43Sc has a half-life of 3.9 hours, so every four hours more than half the radioactivity is lost. It must be used in a positron emission tomography (PET) scan the same day it is made.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
X-ray therapy systems developer Xstrahl announced the launch of the new SARRP (small animal radiation research platform) beamline pre-clinical radiation research and computed tomography (CT) imaging platform. The system was launched at the 2019 International Congress of Radiation Research (ICRR), Aug. 25-29 in Manchester, U.K.
A new 3-D magnetic resonance imaging (MRI) computing technique developed by scientists in WMG at the University of Warwick focuses on hierarchical template matching (HTM) to diagnose cardiac disease without the use of gadolinium contrast. The technique is explored in an article in the journal Scientific Reports.
UCHealth Greeley Hospital of Greeley, Colo., recently became the first healthcare facility in the United States to adopt the Mobilett Elara Max mobile X-ray system from Siemens Healthineers. The Mobilett Elara Max enables comprehensive information technology (IT) security as well as secure system integration into the hospital’s IT environment. UCHealth Greeley has installed two Mobilett Elara Max systems: a standard model and a pediatric-friendly version featuring a giraffe design.UCHealth Greeley Hospital of Greeley, Colo., recently became the first healthcare facility in the United States to adopt the Mobilett Elara Max mobile X-ray system from Siemens Healthineers. The Mobilett Elara Max enables comprehensive information technology (IT) security as well as secure system integration into the hospital’s IT environment. UCHealth Greeley has installed two Mobilett Elara Max systems: a standard model and a pediatric-friendly version featuring a giraffe design.
Fujifilm Medical Systems U.S.A. Inc. recently announced that Ashley County Medical Center (Crossett, Ark.) has invested in new upgrades to its Fujifilm Aspire Cristalle digital mammography solution. Fujifilm upgraded Ashley County Medical Center’s Aspire Cristalle unit with digital breast tomosynthesis (DBT), making it the first Fujifilm 3-D digital mammography solution to be installed in Arkansas.
August 27, 2019 — Medical data delivery company Royal Solutions has partnered with ZipRad to streamline imaging exam ...
Radiation dosimetry company announced plans to exhibit its full range of radiation therapy dosimetry solutions for acceptance, commissioning, beam data acquisition and patient-specific quality assurance (QA) at the 2019 American Society for Radiation Oncology (ASTRO) annual meeting, Sept. 15-19 in Chicago.
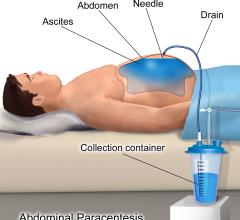
A new Harvey L. Neiman Health Policy Institute study assesses temporal and patient-level differences in paracentesis and thoracentesis procedures performed on Medicare beneficiaries by radiologists and non-radiologists with respect to overall procedure volume, day of week and patient complexity. The study is published online in Journal of Vascular and Interventional Radiology.
Ultrasound imaging company Terason has partnered with DiA Imaging Analysis, provider of artificial intelligence (AI)-based solutions for ultrasound analysis, to provide its cardiac solutions on Terason's point-of-care ultrasound devices.


 September 03, 2019
September 03, 2019