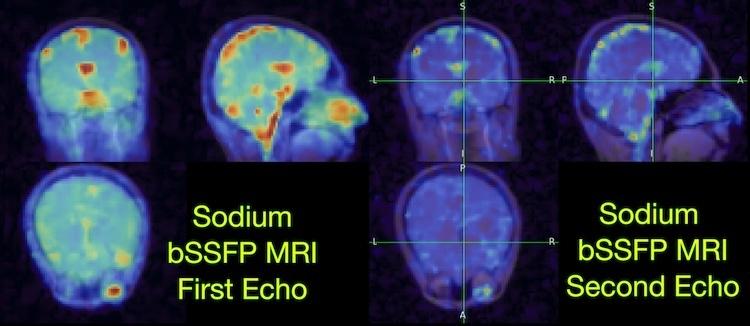
Healthcare has reached a critical juncture.
Magnetic Resonance Imaging (MRI)
MRI creates images from the magnetic resonance created in hydrogen atoms when they are polarized and an electromagnetic pulse is used to knock them off axis. This section includes MR analysis software, MRI scanners, gadolinium contrast agents and related magnetic resonance imaging accessories.
Dec. 9, 2025 — NVision Imaging Technologies, developer of quantum technology enabling imaging of cell metabolism through ...
Dec. 1, 2025 — Researchers at the University of California, Berkeley and University of California, San Francisco have ...
Healthcare has reached a critical juncture. The World Economic Forum estimates that global medical costs will see double ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
Nov. 30, 2025 — GE HealthCare has announced the 510(k) submissions to the U.S. Food and Drug Administration (FDA) ...
Nov.26, 2025 – SimonMed Imaging is presenting four scientific abstracts at RSNA 2025. The presentations acknowledge ...
Nov. 30, 2025 – Metrasens, a provider of ferromagnetic detection systems for MRI safety, has launched Metrasens Vantage ...
In June, the Philips Radiology Experience Tour hit the road to provide healthcare professionals with an opportunity to ...
Nov. 20, 2025 — Avatar Medical and Barco have launched Eonis Vision, marking a new evolution in how medical imaging is ...
Nov. 19, 2025 — Royal Philips has announced an extended partnership with Cortechs.ai. Together, the companies will ...
Nov. 10, 2025 — There has been substantial progress in the past few years in the field of MRI in general and remote MR ...
This summer, the Philips Radiology Experience Tour has been bringing Philips imaging modalities directly to the ...
Once viewed as a solution for after-hours coverage, teleradiology is rapidly expanding into a critical part of radiology ...
Oct. 28, 2025 — Bronchopulmonary dysplasia (BPD) is the most common — and most serious — complication of extreme ...
Oct. 16, 2025 — A strategic collaboration between UC San Diego Health and GE HealthCare will focus on bringing advanced ...
The healthcare industry faces many different types of obstacles in today’s challenging marketplace. Staff shortages ...
Oct. 14, 2025 — Florida Atlantic University has expanded its NeuroInnovate Center, becoming the first institution ...
Oct. 6, 2025 — Hyperfine, Inc. has launched the Portable Ultra-Low-Field Scientific Exchange (PULSE), a subscription ...
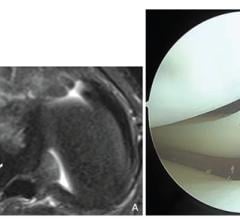
September 24, 2025—According to the American Journal of Roentgenology (AJR), MRI can reliably identify lateral meniscal ...
Sept. 26, 2025 — At the American Society for Radiation Oncology (ASTRO) 2025 annual meeting in San Francisco, Calif ...
Aug. 29, 2025 - Cerebriu recently announced it had received the CE mark for its Apollo Smart Protocol as an OEM-embedded ...
Sep. 15, 2025 —ScanLabMR has introduced its Signal-to-Noise Ratio (SNR) calculator. This feature gives MRI technologists ...
Sept. 15, 2025 — Cook Medical and Siemens Healthineers have announced a strategic commercial partnership aimed at ...
Sept. 10, 2025 —GE HealthCare announced it has entered into an agreement to acquire icometrix, a company focused on ...





 December 11, 2025
December 11, 2025