
Figure 1: Dual-echo sodium balanced steady-state free precession (bSSFP) PETALUTE acquisition on a 3T Siemens Prisma system. Left: First-echo sodium bSSFP PETALUTE images highlighting short-T₂ sodium components and higher signal intensity in regions with rapid transverse relaxation. Right: Second-echo PETALUTE sodium images demonstrating reduced short-T₂ contribution and enhanced long-T₂ contrast. Multi-planar views (coronal, sagittal, axial) illustrate echo-dependent sodium signal distribution across the brain. Image: University of North Carolina at Chapel Hill’s Biomedical Research Imaging Center, Chapel Hill, N.C.
Healthcare has reached a critical juncture. The World Economic Forum estimates that global medical costs will see double digit increases for the third consecutive year since 2023, with estimates suggesting an average rise of 10.4 percent worldwide in 20251 driven by factors including overwhelmed public healthcare systems and a rising demand for private healthcare.
In parallel, the drug development cycle has become more complex, lengthy, and costly in the fight against considerably more complicated illnesses than in the past2. This growing burden underscores the urgent need for advanced technologies that can detect early microstructural and biochemical changes before clinical symptoms emerge.
Established technologies like magnetic resonance imaging (MRI) are moving beyond their traditional realm of disease diagnostics to become a powerful tool in preclinical science for investigating molecular and cellular mechanisms in living organisms.
This article explores a collaboration between Purdue University, the University of North Carolina at Chapel Hill’s Biomedical Research Imaging Center (BRIC), and the University of Cambridge to advance the capabilities of preclinical MRI through the development and validation of novel acquisition strategies — notably PETALUTE, a three-dimensional (3D) dual-echo ultrashort echo time (UTE) sequence with a novel rosette petal trajectory.
Advances in MRI to Support Brain Health
The long-established clinical diagnostic and preclinical basic research role of MRI is changing. Preclinical imaging is increasingly being incorporated into new application fields, providing a valuable link between molecular mechanisms and translational results.
Advances in high and ultra-high field strength MRI have expanded the noninvasive study of neurotransmitter dynamics, microstructure and energy metabolism in the brain — and these developments are accelerating scientific discovery in epilepsy, neurodegenerative disease, brain tumors, and conditions involving autonomic and neuroendocrine dysfunction by enabling functional, structural and metabolic imaging within a single modality.
The PETALUTE sequence is a novel method that helps accelerate MRI by enabling quantitative, artifact-resistant imaging at the molecular and cellular levels. The development and validation of the PETALUTE acquisition strategy for high-resolution, multi-contrast imaging of both 1H and X-nuclei signal components enhances sensitivity, minimizes artifacts and facilitates detailed visualization of short-transverse relaxation time species and metabolic processes crucial to translational neuroscience and disease modeling (Figure 1). It combines multi-nuclear compatibility, UTE, and robust self-gating to build a versatile platform for examining tissue microstructure, ionic environments, and dynamic metabolic activities.
Redefining Image Capture
PETALUTE was developed to overcome limitations in UTE MRI for molecular imaging by improving signal-to-noise ratio (SNR), spatial resolution, and acquisition speed for both 1H-and X-nuclei based metabolic imaging.
Traditional molecular MRI and MR spectroscopic imaging techniques face a choice between spatial resolution quality, scan time, and sensitivity, especially when targeting low-abundance metabolites, rapidly decaying signals, or non-proton nuclei. Single-voxel or localized spectroscopic methods typically require lengthy acquisition times and have limited spatial coverage and conventional techniques may lose quantification accuracy and anatomical detail by unnecessarily sampling central or peripheral regions of k-space.
The multi-echo UTE design of the PETALUTE sequence, a novel 3D dual-echo UTE MRI sequence, addresses this challenge. A modified rosette k-space trajectory strategically increases sampling density in both the center and the edges of k-space, allowing efficient volumetric imaging of short-T2* species across various nuclei for improved SNR and spatial accuracy. Each petal readout starts and ends at the k-space center, allowing two or more echoes per repetition time and flexible, interleaved contrast generation, quantitative T2* mapping, and compressed sensing–accelerated reconstruction (Figure 2).
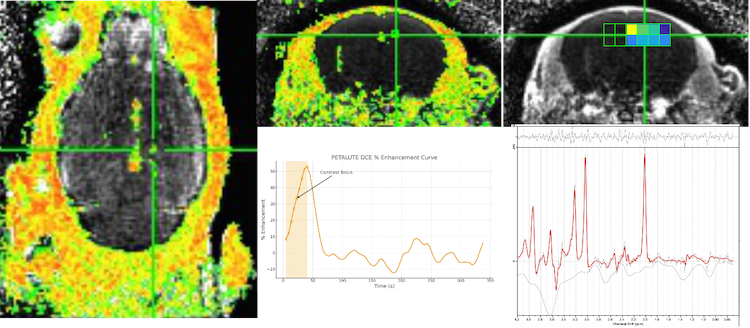
The PETALUTE sequence on a Bruker preclinical MRI platform demonstrated significant improvements in imaging efficiency and accuracy (Figure 3), enabling high-resolution volumetric imaging within a short scan time while maintaining sensitivity to rapidly decaying signals. By capturing sub-voxel contrast details across large tissue volumes, PETALUTE supports comprehensive evaluation of microstructural and metabolic changes with strong translational relevance for neurological, musculoskeletal, and oncological studies.
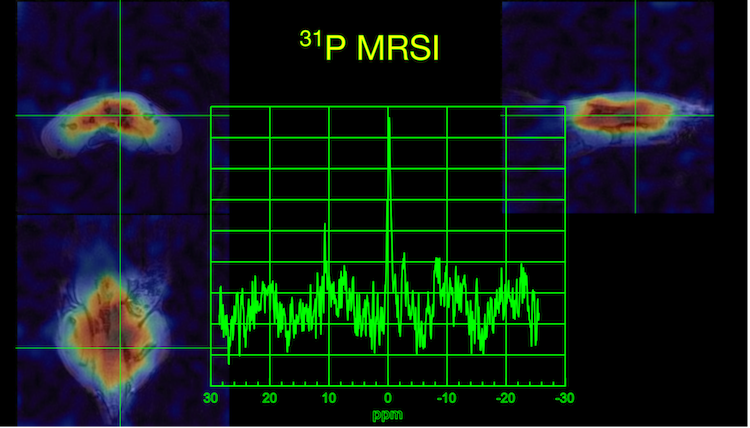
[i] Booth, S., Song, S., Wang, T.W. et al. PETALUTE fMRI in the mouse brain. Proceedings 33rd Scientific Meeting, ISMRM (2025)
Supporting Neurological Research
A 2023 study4 demonstrates PETALUTE in capturing often inaccessible signal components with ultrashort transverse relaxation times (µT2) in brain tissue. Because standard gradient-echo and spin-echo sequences are inherently biased toward long T2 components, poor sensitivity for rapidly decaying structures can hamper analyses.
Enter PETALUTE: its fast echo times (in the tens of microseconds) enable direct imaging of short-T2* species for applications including visualizing the myelin bilayer, which cannot be captured by traditional MRI sequences due to its rapid delay. PETALUTE’s multi-echo UTE framework, combined with center-out rosette sampling, enables detection and spatial localization of myelin signal components.
Potential in Musculoskeletal Imaging
The PETALUTE sequence demonstrates significant translational potential across musculoskeletal and abdominal imaging, especially in cartilage, bone, and liver tissues where its UTE component enables the direct detection of short-T2* components that are typically invisible to conventional sequences.
One key application is osteoarthritis, which involves glycosaminoglycan (GAG) loss from cartilage. Sodium (23Na) MRI provides a noninvasive way to measure GAG content, but faces challenges like long scan times and low spatial resolution. PETALUTE enables in vivo sodium imaging at 3T with accuracy comparable to standard methods while reducing scan time by 41% and enhancing spatial detail in thin cartilage layers5.
A New Future of Imaging
The potential for PETALUTE to drive discoveries in clinical and preclinical imaging is increasingly evident. As its adoption expands across the imaging research community, PETALUTE is being integrated into a growing range of applications. With robust preclinical results and proven clinical feasibility, PETALUTE bridges the gap between basic science and clinical practice, positioning it as a next-generation framework for both early-phase clinical studies and mechanistic research in animal models.
Uzay Emir is an associate professor in the University of North Carolina Department of Radiology with a joint appointment in the Department of Biomedical Engineering, and a member of the Biomedical Research Imaging Center, as an associate professor from Purdue University. Dr.Stephen Sawiak is a senior research associate and Fellow of Fitzwilliam College, University of Cambridge.
References
- World Economic Forum, Health and Healthcare Systems, 2025 World Economic Forum Annual Meeting. https://www.weforum.org/stories/2025/01/health-technology-global-health…
- Chopra H, Annu, Shin DK, Munjal K, Priyanka, Dhama K, Emran TB. Revolutionizing clinical trials: the role of AI in accelerating medical breakthroughs. Int J Surg. 2023 Dec 1;109(12):4211-4220. doi: 10.1097/JS9.0000000000000705. PMID: 38259001; PMCID: PMC10720846. https://pmc.ncbi.nlm.nih.gov/articles/PMC10720846/
- Booth, S., Song, S., Wang, T.W. et al. PETALUTE fMRI in the mouse brain. Proceedings 33rd Scientific Meeting, ISMRM (2025)
- Shen, X., Özen, A.C., Sunjar, A. et al. Ultra-short T2 components imaging of the whole brain using 3D dual-echo UTE MRI with rosette k-space pattern. Magn Reson Med 89, 508-521 (2023) https://doi.org/10.1002/mrm.29451
- Villarreal, C.X., Shen, X., Alhulail, A.A. et al. An accelerated PETALUTE MRI sequence for in vivo quantification of sodium content in human articular cartilage at 3T. Skeletal Radiol (2024)


 February 27, 2026
February 27, 2026 









