DrChrono Inc. and 3D4Medical have teamed up so practices across the United States can access 3-D interactive modeling and animation videos from within their electronic health record (EHR) to better educate patients.
A new study positron emission tomography (PET) scans has identified a biomarker that may accurately predict which patients with one type of HER2-positive breast cancer might best benefit from standalone HER2-targeted agents, without the need for standard chemotherapy. The study was conducted by researchers at the Johns Hopkins Kimmel Cancer Center in an effort to further individualize therapy and avoid over-treating patients.
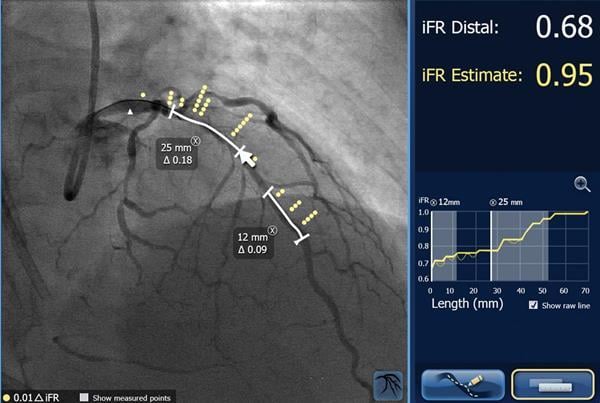
As many as one in four patients who undergo cath lab interventions can benefit from a technology that identifies the ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Brain magnetic resonance imaging (MRI) without contrast agent is just as effective as the contrast-enhanced approach for monitoring disease progression in patients with multiple sclerosis (MS), according to a new study in the journal Radiology. The findings support the possibility that contrast enhancement can be omitted from routine follow-up scans.
Virtual reality (VR) and its less immersive kin, augmented reality (AR), are gaining traction in some medical ...
Machine learning is already having an enormous impact on cardiology, automatically calculating measurements in ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Calcium is a critical signaling molecule for most cells, and it is especially important in neurons. Imaging calcium in brain cells can reveal how neurons communicate with each other; however, current imaging techniques can only penetrate a few millimeters into the brain.
Fujifilm Medical Systems USA announced it has fulfilled all U.S. Food and Drug Administration (FDA) regulatory requirements for three new image processing and software advancements for its digital mammography system, Aspire Cristalle. Specifically, S-View, Iterative Super-resolution Reconstruction (ISR) and Tomosynthesis Spot — innovative tools designed to meet the dual challenge of higher image quality and lower dose — are now commercially available in the United States.
As a VNA, GE Healthcare Centricity Clinical Archive weaves together data from many different sources and systems. The ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Artificial intelligence (AI) company Bay Labs announced the presentation of two studies assessing performance of the company’s deep learning software for cardiovascular imaging. The first evaluated the software when used by medical professionals with no prior ultrasound experience to acquire diagnostic-quality echocardiograms, and the second evaluated the fully automated calculation of ejection fraction (EF) with accuracy and increased reproducibility. Results from these studies will be presented at the American College of Cardiology (ACC) 68th Annual Scientific Session, March 16-18 in New Orleans.
Canon Medical Systems Europe B.V. introduced the all-new Aquilion Start computed tomography (CT) system to the European market at the 2019 European Congress of Radiology (ECR), Feb. 28-March 3 in Vienna, Austria. Featuring premium technologies based on Canon Medical’s Aquilion family, the new Aquilion Start will provide the opportunity for medical institutions to extend their radiology practice.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Hologic Inc. announced it has received a CE mark in Europe for its Omni hysteroscope, a three-in-one modular scope with advanced visualization capabilities designed for both diagnostic and therapeutic hysteroscopic procedures. Obstetricians and gynecologists (ObGyns) can use the new Omni hysteroscope in out- and inpatient settings.
A team of researchers at the University of Washington announced they developed a new automated system for producing zirconium-89, a diagnostic radionuclide used for cancer tumor imaging.
Siemens Healthineers will introduce the Somatom go.Top Cardiovascular Edition, a new version of its established computed tomography (CT) system, at the 68th Annual Scientific Session and Expo of the American College of Cardiology (ACC), March 16-18 in New Orleans. The Somatom Go.Top Cardiovascular Edition is designed to deliver personalized patient dose control in all types of routine cardiovascular imaging. The cost-effective, 128-slice scanner offers outpatient cardiology offices as well as hospitals access to not just coronary CT angiography (CCTA), but also advanced tests, including the HeartFlow FFRCT (fractional flow reserve computed tomography) Analysis.
The number of new particle therapy rooms ordered worldwide dropped by almost 20 percent in 2018, according to a new report from MEDraysintell, continuing a trend dating back to mid-2016. The report says this trend may change if particle therapy vendors are able to build a strong order book in 2019 and 2020.
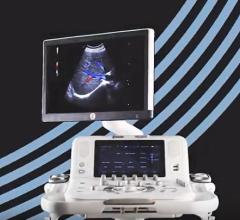
Esaote announced the launch of MyLab X8, a high-performance, versatile ultrasound platform to support hospitals and clinics facing today’s challenges, at the 2019 European Congress of Radiology (ECR), Feb. 27-March 3 in Vienna, Austria.
An imaging procedure commonly performed before starting cancer treatment can provide valuable clues about a patient's risk for heart problems in the months and years after treatment. However, this information is not always reported and is rarely acted upon in current practice, according to research being presented at the American College of Cardiology's 68th Annual Scientific Session, March 16-18 in New Orleans.
At RSNA 2018, iCad showed how its ProFound AI for digital breast tomosynthesis technology might help in the ...
Genomic medicine company Lucence Diagnostics announced a new project to develop artificial intelligence (AI) algorithms for improving diagnosis and treatment of liver cancer. The goal is to combine the imaging and molecular data from liver cancer patients into smarter software tools that help physicians make better treatment decisions.Genomic medicine company Lucence Diagnostics announced a new project to develop artificial intelligence (AI) algorithms for improving diagnosis and treatment of liver cancer. The goal is to combine the imaging and molecular data from liver cancer patients into smarter software tools that help physicians make better treatment decisions.

 March 18, 2019
March 18, 2019