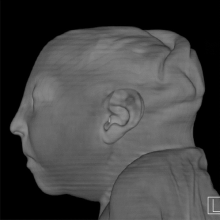
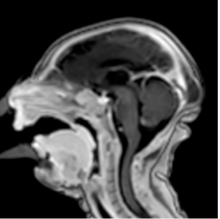
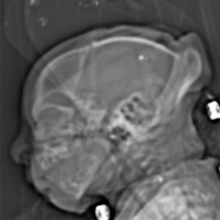
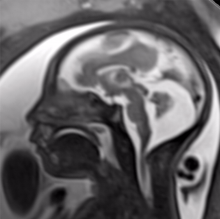
August 26, 2016 — The latest version of Elekta’s Monaco treatment planning software was recently highlighted in multiple presentations at the 58 th American Association of Physicists in Medicine (AAPM) annual meeting and exhibition, July 31-Aug. 4 in Washington, D.C.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now