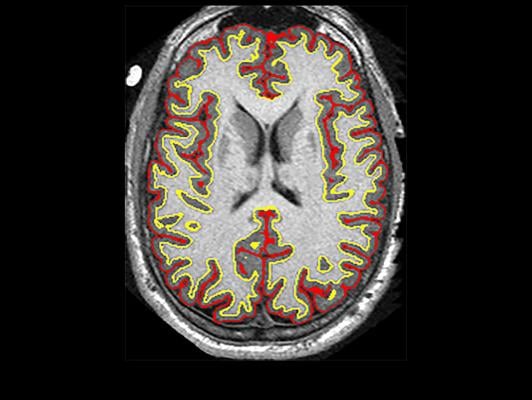
An NIH-funded clinical trial suggested that the anti-inflammatory drug ibudilast may slow brain shrinkage caused by progressive MS. Image courtesy of Robert J. Fox, M.D., Cleveland Clinic.
August 30, 2018 — Results from a clinical trial of more than 250 participants with progressive multiple sclerosis (MS) revealed the drug ibudilast was better than a placebo in slowing down brain shrinkage. The study also showed that the main side effects of ibudilast were gastrointestinal and headaches.
The study was supported by the National Institute of Neurological Disorders and Stroke (NINDS), part of the National Institutes of Health, and published in the New England Journal of Medicine.1
“These findings provide a glimmer of hope for people with a form of multiple sclerosis that causes long-term disability but does not have many treatment options,” said Walter J. Koroshetz, M.D., director of the NINDS.
MS occurs when there is a breakdown of myelin, a fatty white substance wrapped around axons, which are long strands that carry messages from and between brain cells. When myelin starts to break down, communication between brain cells slows down, leading to muscle weakness and problems with movement, balance, sensation and vision. MS can be relapsing-remitting, in which symptoms occur then disappear for weeks or months and then may reappear, or progressive, which is marked by a gradual decline in function.
Robert J. Fox, M.D., a neurologist at Cleveland Clinic in Ohio, led a team of researchers across 28 clinical sites in a brain imaging study. They were investigating whether ibudilast was better than placebo in reducing the progression of brain atrophy, or shrinkage, in patients with progressive multiple sclerosis.
In the study, 255 patients were randomized to take up to 10 capsules of ibudilast or placebo per day for 96 weeks. Every six months, the participants underwent magnetic resonance imaging (MRI) brain scans. Fox’s team applied a variety of analysis techniques on the MRI images to assess differences in brain changes between the two groups.
The study showed that ibudilast slowed down the rate of brain atrophy compared to placebo. Fox and his colleagues discovered that there was a difference in brain shrinkage of 0.0009 units of atrophy per year between the two groups, which translates to approximately 2.5 milliliters of brain tissue. In other words, although both groups experienced atrophy, the brains of the patients in the placebo group shrank on average 2.5 milliliters more over two years compared to the ibudilast group. The whole adult human brain has a volume of approximately 1,350 milliliters. However, it is unknown whether that difference had an effect on symptoms or loss of function.
There was no significant difference between the groups in the number of patients who reported adverse effects. The most common side effects associated with ibudilast were gastrointestinal, including nausea and diarrhea, as well as headaches and depression.
“The trial’s results are very encouraging and point towards a potential new therapy to help people with progressive MS,” said Fox. “It also increased our understanding of advanced imaging techniques, so that future studies may require a smaller number of patients followed over a shorter period of time. This leads to increased efficiency of clinical research. These imaging methods may also be relevant to a host of other neurological disorders.”
Future research will test whether reducing brain shrinkage affects thinking, walking and other problems in people with MS. In addition, future studies will examine whether ibudilast slows the progression of disability in MS patients.
For more information: www.nejm.org
Reference


 February 13, 2026
February 13, 2026 









