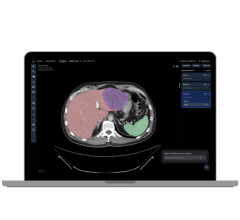
May 9, 2018 — Body Vision Medical received clearance from the U.S. Food and Drug Administration (FDA) to market their LungVision Tool. The LungVision Tool is an affordable lung navigation catheter with what the company calls superb maneuverability. It is used in conjunction with standard bronchoscopes and the LungVision System to guide endotherapy accessories to small pulmonary nodules.
This approval clears the way for Body Vision Medical to accelerate commercialization efforts for its LungVision Platform for pulmonary specialists across the U.S., providing an affordable and effective real-time solution for early-stage lung cancer diagnostics and treatment procedures.
The LungVison Imaging and Navigation System was cleared by the FDA in May 2017 and has been successfully used in over 290 clinical procedures in 10 lung cancer centers across the U.S.
"Body Vision has pioneered a new generation platform for navigation bronchoscopy that applies an augmented reality approach to plan, visualize and accurately track radiolucent bronchial nodules in real time," said Krish Bhadra, M.D., from CHI Memorial Hospital, Chattanooga, Tenn. "The LungVision navigation tool has clear performance and cost benefits over current navigation tools that are bound to electromagnetic sensing."
The clinical experience from a multicentral study with the LungVision System will be presented at the American Thoracic Society Conference, May 18-23 in San Diego.
For more information: www.bodyvisionmedical.com


 February 11, 2026
February 11, 2026