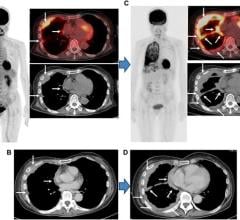
Oct. 30, 2025 — Sirona Medical has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Sirona Advanced Imaging Suite, marking a significant regulatory milestone and the company's first Class II medical device designation.
PET-CT
PET-CT combines positron emission tomography (PET) detectors and computed tomography (CT) into one imaging system.
Oct. 30, 2025 — Sirona Medical has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Sirona ...
Oct. 6, 2025 — GE HealthCare has announced a strategic collaboration with Erasmus MC University Medical Center (Erasmus ...
Aug. 13, 2025 — A new study from Denmark shows for the first time that men with biochemically recurrent prostate cancer ...
The healthcare industry faces many different types of obstacles in today’s challenging marketplace. Staff shortages ...
Aug. 5, 2025 — New Lantern has announced the launch of two specialized viewer modes: the Mammography Viewer Mode and PET ...
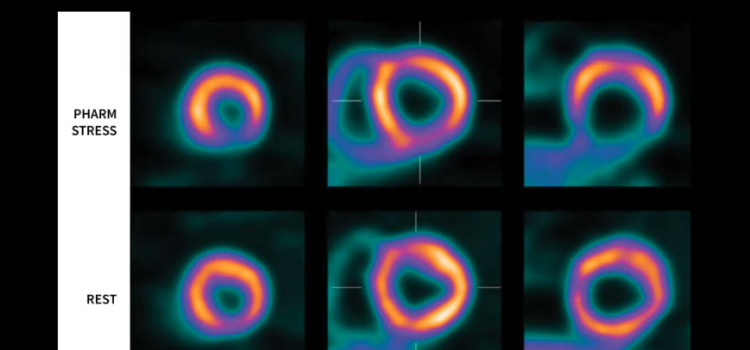
June 23, 2025 — GE HealthCare’s commitment to advancing precision care in cardiology through its molecular imaging ...
June 19, 2025 — Building on a collaboration that spans more than three decades, GE HealthCare has renewed its research ...
Reporting produces the tangible work product of diagnostic radiologists. Reports should reflect the expertise of the ...
Nov. 25, 2024 — GE HealthCare has announced it is woking with Peter MacCallum Cancer Centre in Melbourne, Australia, to ...
July 31, 2024 — In a head-to-head comparison with FDG PET/CT, FDG PET/MRI demonstrated comparable or superior diagnostic ...
July 25, 2024 — Positron Corporation, a leading molecular imaging medical device company offering PET & PET-CT imaging ...
People who live in rural America may deserve the same quality healthcare as anyone living in the U.S., but it is not ...
July 16, 2024 — A new research paper was published in Oncotarget's Volume 15 on June 20, 2024, titled, “Comparison of ...
July 2, 2024 — A new editorial paper was published in Oncoscience (Volume 11) on May 20, 2024, entitled, “Deep learning ...
June 18, 2024 — Positron Corporation, a leading molecular imaging medical device company offering PET and PET-CT ...
Technological advancements in positron emission tomography/computed tomography (PET/CT) offer both clinicians and ...
June 14, 2024 — Positron Corporation, a leading molecular imaging medical device company offering PET and PET-CT ...
June 13, 2024 — Positron Corporation, a leading molecular imaging medical device company offering PET and PET-CT ...
June 10, 2024 — Siemens Healthineers announces the Food and Drug Administration clearance of the Biograph Trinion, a ...
May 14, 2024 — University Hospitals (UH) and Siemens Healthineers announce a 10-year strategic alliance that builds on ...
April 23, 2024 — CDL Nuclear Technologies, a pioneer in advanced diagnostic solutions, is proud to announce the launch ...
January 23, 2024 — Siemens Healthineers announces the Food and Drug Administration (FDA) clearance of syngo Virtual ...
January 23, 2024 — Quibim announced it has added an industry-leading cancer detection capability to its prostate tool ...
According to research conducted by Polaris Market Research, the global positron emission tomography (PET)/computed ...





 October 30, 2025
October 30, 2025