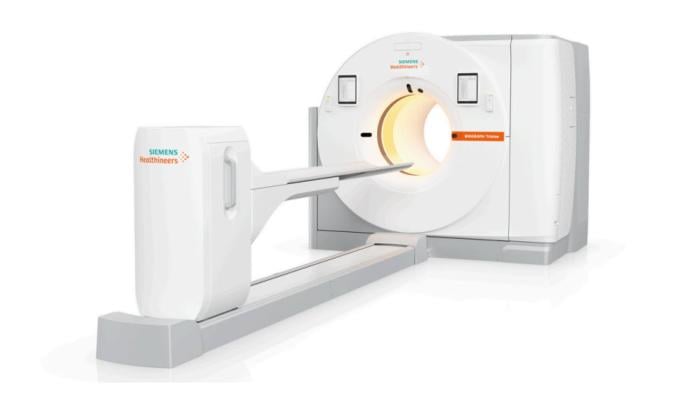
June 10, 2024 — Siemens Healthineers announces the Food and Drug Administration clearance of the Biograph Trinion, a high-performance, energy-efficient positron emission tomography/computed tomography (PET/CT) scanner with a wide range of clinical capabilities and a low lifetime operational cost.
“With the Biograph Trinion, Siemens Healthineers is proud to offer customers a high-performance PET/CT scanner that delivers the precision and speed needed for clinical demands,” said James Williams, PhD, head of Molecular Imaging at Siemens Healthineers. “This new system is designed to be user- and patient focused as well as a sustainable investment in terms of reduced installation and operational costs and easy, on-site scalability.”
The scanner’s high-performance PET/CT platform has a new, scalable, air-cooled, digital detector based on lutetium oxyorthosilicate (LSO) crystal elements. This detector delivers high spatial resolution and an ultrafast time-of-flight¹ performance of 239 picoseconds for small lesion detectability and effective sensitivity up to 128 cps/kBq, enabling fast scans and low patient radiation doses. CT technology migrated from the company’s established SOMATOM go. platform provides fast, low-dose, high-quality CT scanning with up to 128 slices. The scanner’s single, integrated platform enables consistent PET, CT, and post-processing workflows on one system without the need for a separate CT acquisition system or post-processing solution. Integrated post-processing imaging applications include oncology, theranostics (which combines the approaches of diagnostics and therapeutics to identify and treat certain forms of cancer), cardiovascular imaging, and neurology imaging (including Alzheimer’s disease).
The Biograph Trinion is designed for an optimal user and patient experience. Its workflow, which is powered by artificial intelligence and enabled by the myExam Companion intelligent user interface, automates tasks and guides the user through each step of the exam for fast, consistent results. The mobile workflow enabled by the portable Scan&Go tablet and remote control allows the user to quickly and more easily set up scanning procedures and choose whether to operate the scanner either in the room or from outside the room. An integrated patient camera in the rear of the gantry permits the user to monitor and communicate with the patient, and the patient moodlight feature can help alleviate stress.
The scanner platform is designed to be sustainable with a low total cost of ownership. Its air-cooling apparatus is easier to install than the chiller required for a water-cooled PET/CT scanner. The scanner’s compact footprint allows it to fit into traditional PET/CT rooms, and unlike a traditional PET/CT scanner that requires three dedicated rooms for the workstation, scanner, and computer equipment, the Biograph Trinion integrates its computer technology inside the system gantry, eliminating the need for an equipment/utility room. The scanner is designed to be energy-efficient, with a smart power-save mode that powers down the system overnight and automatically powers it up the next morning without disrupting workflow, for energy savings of up to 46%. The Biograph Trinion‘s scalable axial field of view can be upgraded on site with the latest innovations and technology without costly room renovations or impeding workflow.
For more information: www.siemens-healthineers.com
Reference:
1 Ultra-fast time of flight (TOF) is defined as less than 275 picoseconds (ps). Data on file.


 March 04, 2026
March 04, 2026 









