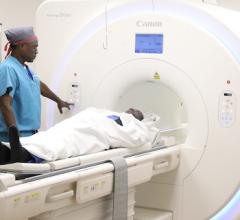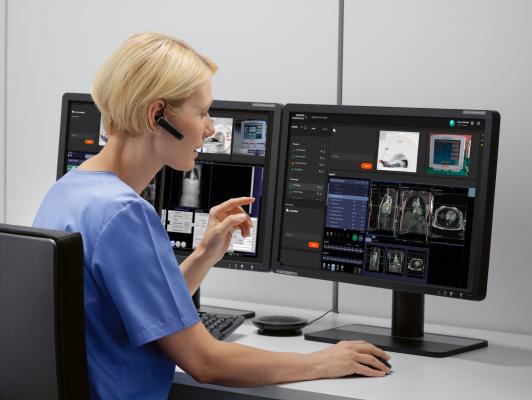
January 23, 2024 — Siemens Healthineers announces the Food and Drug Administration (FDA) clearance of syngo Virtual Cockpit, a private, secure communication platform for real-time image visualization, acquisition, and collaboration between healthcare professionals across multiple sites. The software enables users to connect to computed tomography (CT), magnetic resonance (MR), positron emission tomography (PET), single-photon emission CT (SPECT), PET/CT, SPECT/CT, and PET/MR scanners from Siemens Healthineers as well as other equipment vendors, regardless of location. syngo Virtual Cockpit is the first and only multi-vendor remote scanning software to receive FDA clearance as a medical product.
With syngo Virtual Cockpit, radiologic technologists can use live audio, video, and chat functionalities to conduct scans or provide support for up to three different remote scanners simultaneously. By deploying technologists across multiple locations in this manner, healthcare providers can address the ongoing technologist shortage as well as standardize care and achieve diagnostic consistency across the health system. This standardization can lead to a more accurate patient diagnosis. In addition to enabling offsite scanning and scan support, syngo Virtual Cockpit can be used to provide on-the-job staff training at remote facilities. Also, by increasing the local availability of advanced imaging, syngo Virtual Cockpit can benefit patients in suburban and rural settings who would otherwise need to travel long distances for more complex scans.
“With this FDA clearance for syngo Virtual Cockpit, Siemens Healthineers proudly offers our customers the first and only multi-vendor, multi-modality, FDA-cleared solution for remote assistance scanning,” said Peter Shen, head of digital and automation at Siemens Healthineers North America. “This FDA clearance means that our customers can use syngo Virtual Cockpit for their remote scanning operations with even more confidence that they are using a proven solution—one that prioritizes patient safety and convenience while also addressing operational and staffing challenges.”
syngo Virtual Cockpit is an FDA-cleared medical device not yet commercially available in all countries, and the service offering cannot be guaranteed due to regulatory or other reasons. Please contact your local Siemens Healthineers organization for further details.
For more information: siemens-healthineers.us/syngo-virtual-cockpit


 March 06, 2026
March 06, 2026