According to research conducted by Polaris Market Research, the global positron emission tomography (PET)/computed tomography (CT) scanner device market size is expected to reach $3.34 B by 2028. The report, PET/CT Scanner Device Market Share, Size, Trends, Industry Analysis Report; Segment Forecast, 2021 – 2028, gives detailed insight into current market dynamics and provides analysis on future market growth.
CT imaging produces images of internal organs using X-ray systems and contrast agents, which aid in the interpretation of organ function. When comparing PET and CT scans separately, the combined PET/CT scans provide images of the precise location of irregular metabolic activity within the body, leading to a more reliable diagnosis. The factors driving the growth of the PET/CT market globally are technological developments in PET imaging, such as the advent of integrated imaging systems, with radiation therapy leading to medical revolution. In 2023, several new PET/CT systems were introduced to the market.
New Product Introductions
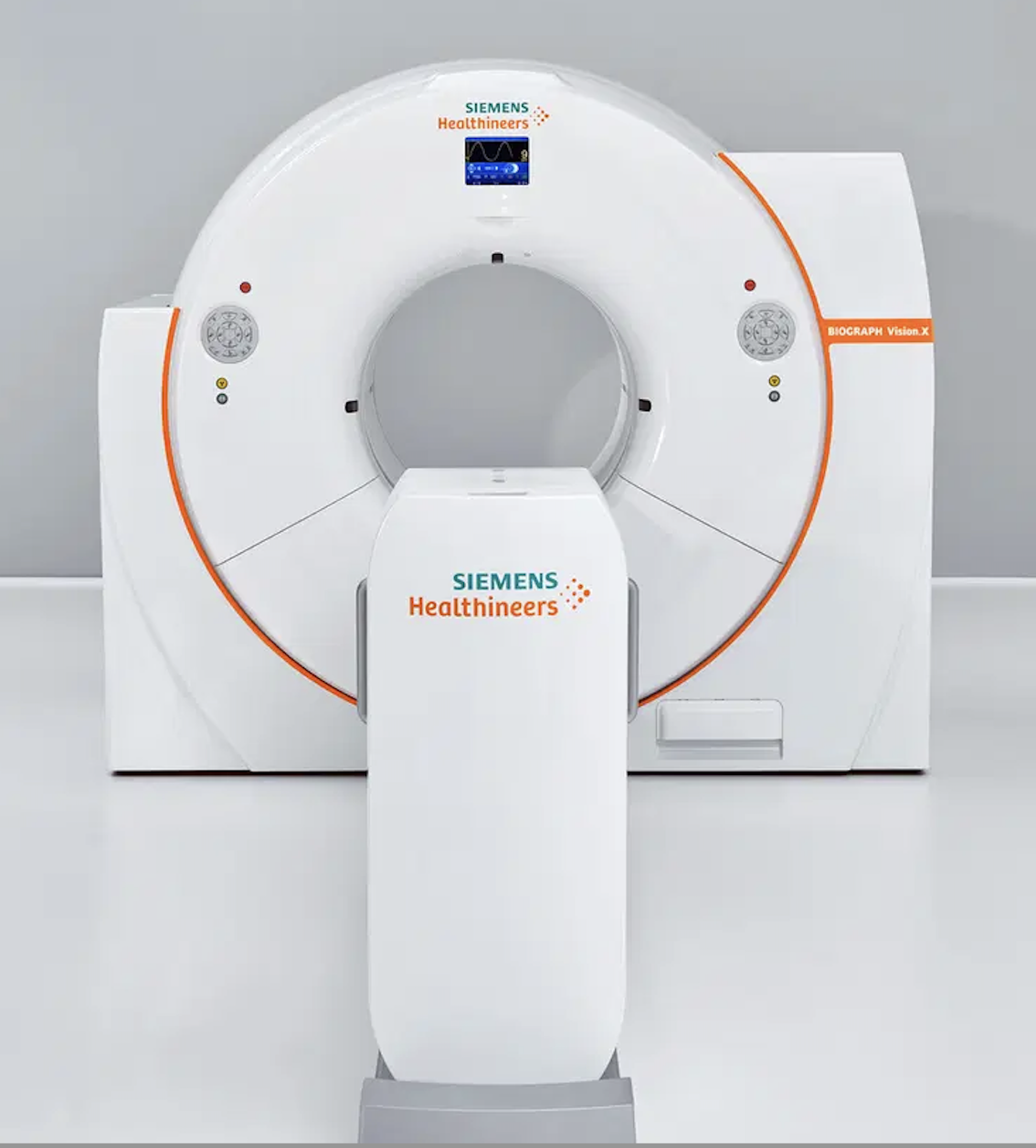
Siemens Healthineers. In November 2023, Siemens Healthineers announced that its Biograph Vision.X system received US Food and Drug Administration (FDA) clearance. This PET/CT system offers a time of flight (TOF) of 178 picoseconds, and builds on the established performance of the Biograph Vision family of scanners. Its Optiso Ultra Dynamic Range (UDR) detector technology contains silicon photomultipliers (SiPMs), which enable the use of small 3.2 mm x 3.2 mm lutetium oxyorthosilicate (LSO) crystal elements. These tiny crystals provide higher spatial resolution than larger crystals. According to the company, leveraging these very small LSO crystals that are 100% covered by SiPMs, the Biograph Vision.X delivers high 48 mm volumetric resolution and an industry-best temporal resolution of 178 ps. For these reasons, the scanner can deliver a 20% performance improvement (TOF gain) that can improve patient throughput as well as reduce patient radiation exposure and radiotracer cost. The scanner’s large 78 cm bore helps calm anxious patients and allows easier positioning of bariatric patients or radiotherapy devices. It fits into any room that houses a PET/CT scanner from the Biograph Vision family.
Xeos. The FDA-cleared Xeos Aura 10 offers PET/CT for the operating room (OR) and presents surgeons and clinicians with an unprecedented level of precision, harnessing the sensitivity of PET imaging with submillimeter spatial resolution. The mobile scanner ensures high-quality images are captured in the OR — 10 minutes after excision — eliminating the need for specimen transport to the radiology or pathology departments during surgery. This advancement promises to transform the landscape of intraoperative diagnostics, offering real-time insights that can make all the difference in patient care.
Conventional PET/CT imaging plays a pivotal role in diagnosis and follow-up for a variety of medical conditions, but there is a growing challenge in healthcare systems worldwide as an increasing volume of complex patients puts pressure on intraoperative efficiency, which impacts the operating room as well as other departments, such as radiology and pathology. The AURA 10 helps surgeons make informed decisions, rapidly verified, on the spot. Use indications could include breast cancer, prostate cancer, and many other types of cancer.
United Imaging. “There has been a debate in the marketplace for a while about the relative importance of resolution, axial field of view (FOV), and time of flight (TOF),” stated Jeffrey Bundy, PhD, CEO of United Imaging Healthcare Solutions about the company’s product launch in a written statement at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) this past June. Bundy added, “With the new wide-bore uMI Panorama and its 2.9 mm NEMA resolution, 35 cm axial FOV and 194 ps timing resolution, we will gladly have a conversation on any single specification or the collective benefit of all of them, because with this particular product, the need to compromise is gone.”
United Imaging showcased its molecular imaging technology platform along with the uMI Panorama, called uExcel, at SNMMI. The platform integrates hardware and software innovations to optimize performance, imaging capabilities and system functionalities. Its ultra-digital platform (UDP) detector boasts a high-performance ASIC chip, and its AI-empowered workflow streamlines operations and enhances examination efficiency. According to the company, exceptional image quality can be achieved with a lower dose, and the quality assurance module maintains peak performance of the system. The uMI Panorama is a family of products that also includes a 510K-pending system with a 148 cm axial FOV, the uMI Panorama GS. Built on the uExcel platform, this ultra-long axial FOV system allows for fast and high-resolution whole-body PET/CT imaging in a single bed position. It also was unveiled at SNMMI.
Partnerships for Growth
Positron Corporation announced that it entered a Novation Agreement with Intelligent Neuclear Medical, a wholly owned subsidiary of Neusoft Medical Systems. Positron has expanded its role as manufacturer with Intelligent Neuclear Medical designated as the company’s contracted components supplier. Positron’s classification as the device manufacturer is significant because it will register with the FDA as the applicant of its FDA 510k submission for Medical Device Clearance for its Affinity PET/CT imaging device. Moving forward, Positron will adhere to all FDA requirements, quality systems regulations, and import regulations. Through its role as manufacturer, Positron will be able to observe and comply with FDA requirements and perform future device and procedural reviews more efficiently.
This article served as an introduction for a comparison chart of specifications for PET/CT Systems. The chart can be accessed at https://www.itnonline.com/chart/contrast-media-injectors. Access requires a login but it is free and only takes a minute to complete. View ITN's PET/CT Systems Comparison Chart here.


 March 09, 2026
March 09, 2026 









