June 3, 2021 — Medical imaging AI specialist Avicenna.AI announced that it has received certification in the United States and European Union for Cina Chest, its new AI solution that leverages deep learning algorithms for emergency triage of deadly vascular conditions.
In addition to securing a CE Mark in the EU, Cina Chest has also received 510(k) clearance from the US Food and Drug Administration (FDA) for its automatic detection and triage capabilities for both pulmonary embolism (PE) and aortic dissection (AD) from CT-scan imaging.
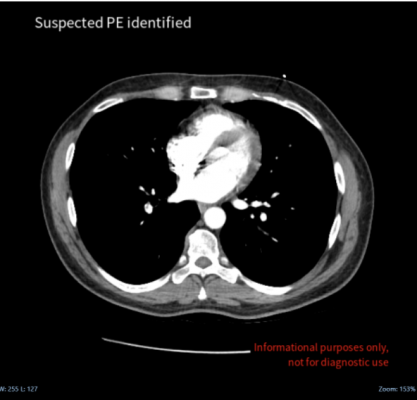
Pulmonary embolism is the blockage of an artery in the lungs and is one of the major causes of death, morbidity and hospitalization worldwide. It is difficult to diagnose PE as it manifests in diverse ways and can be mimicked by a range of other conditions. Cina Chest provides rapid automatic PE detection on CT chest angiography, providing clinicians with accurate and rapid real-time alerts on the disease.
Aortic dissection is a tear in the inner layer of the aorta, allowing blood to flow between the layers. Although relatively rare, AD has a high mortality rate and is often not diagnosed when it first appears. Patients who receive the appropriate treatment in a timely manner have a high survival rate, so the importance of a quick and accurate diagnosis is critical. Cina Chest identifies acute AD cases that require urgent intervention, providing accurate, real-time alerts on thoraco-abdominal CT angiography.
Cina Chest is part of Avicenna’s CINA family of AI tools that support the treatment of emergencies, including CINA HEAD, its FDA-cleared and CE-Marked solution that supports the detection and triage of stroke and neurovascular emergencies.
“At Avicenna, we specialize in the development of AI algorithms that can identify acute abnormalities, and Cina Chest is the latest application we’ve developed to enhance emergency room radiology,” said Cyril Di Grandi, co-founder and CEO of Avicenna.AI. “Our PE and AD triage tools are the third and fourth algorithms we’ve released in less than 12 months, demonstrating our ambition to create AI applications that support detection and triage of emergencies throughout the entire body.”
For more information: www.avicenna.ai


 February 16, 2026
February 16, 2026 









