
Let’s start with an actual clue from a Jeopardy Tournament of Champions match: “The main cause of false negative results with this type of imaging is breast tissue that is highly dense.” Of course, the correct answer is the question: “What is a mammogram?”
Given that until recently there was limited understanding by the general public as to what breast density is — or why it matters — it is encouraging that the topic has reached this level of cultural awareness. Unfortunately, there are still too many women who do not know that breast density could hide cancer on their mammogram; who do not know that breast density carries an increased risk of developing cancer; and who do not know that other screening tools are available that could help detect missed cancers at an earlier stage.
Federal Standardized Dense Breast Notification
That is why DenseBreast-info.org (DB-i) continues to push for the implementation of a national “dense breast” reporting standard. As a reminder, the U.S. Food and Drug Administration (FDA) is advancing a standard for breast density notification for American women and has indicated that this will happen by early 2023.
Until that time, individual state laws determine the level of patient density notification. According to DB-i analysis, 38 states and Washington, DC, currently require some level of density notification; however, the state laws differ significantly in depth and breadth. Six state laws only require a general notification about breast density be provided without actually telling the patient if she, herself, has dense breasts (Conn., La., Md., Mo., N.J. and Texas). Additionally, of the 38 state laws, only about half mention that a discussion about supplemental screening is appropriate and not all mention breast density as an independent risk factor for the development of breast cancer.
Every state that enacts its own ‘inform’ law adds a new version into the mix. Neighboring states may have very different notification requirements. This creates an inequity in actionable information a patient receives based on where she lives.
A national reporting standard is needed in order to ensure all American women receive at least the same basic information. Successful implementation will require medically sourced educational materials such as DenseBreast-info.org, and should both encourage and validate personal screening strategies.
Federal Insurance Legislation
On Dec. 13, 2022, Rep. Rosa DeLauro (Conn.) and Rep. Brian Fitzpatrick (Pa.) introduced federal legislation that would require insurers to cover no-cost additional breast imaging for women with dense breasts or at higher risk for breast cancer. Given DB-i’s experience in bill analysis, Chief Scientific Advisor Wendie Berg, MD, and I were asked to review and offer input on bill language. We are particularly delighted that the DB-i tagline “Find it Early” is being used as the bill’s name!
Currently, post-mammography expanded breast imaging coverage is required by law on a state-by-state basis and varies widely. Fifteen states require some level of expanded coverage for ultrasound and/or magnetic resonance imaging (MRI) and one state for diagnostic breast imaging only.
Note that even with “coverage” required, co-pays and deductibles may still apply. And, even if there is a state law, it does not necessarily apply to all policies within that state. For example, out-of-state plans, self-funded employer plans, and national plans like Medicare may be exempt from state laws. The Find It Early Act seeks to address these exemptions.
However, even if there is no state insurance law, supplemental screening, such as ultrasound or breast MRI for high risk women is generally covered if ordered by a physician, though usually subject to co-pay and deductible. (See Map 1 for state-by-state specific information.)
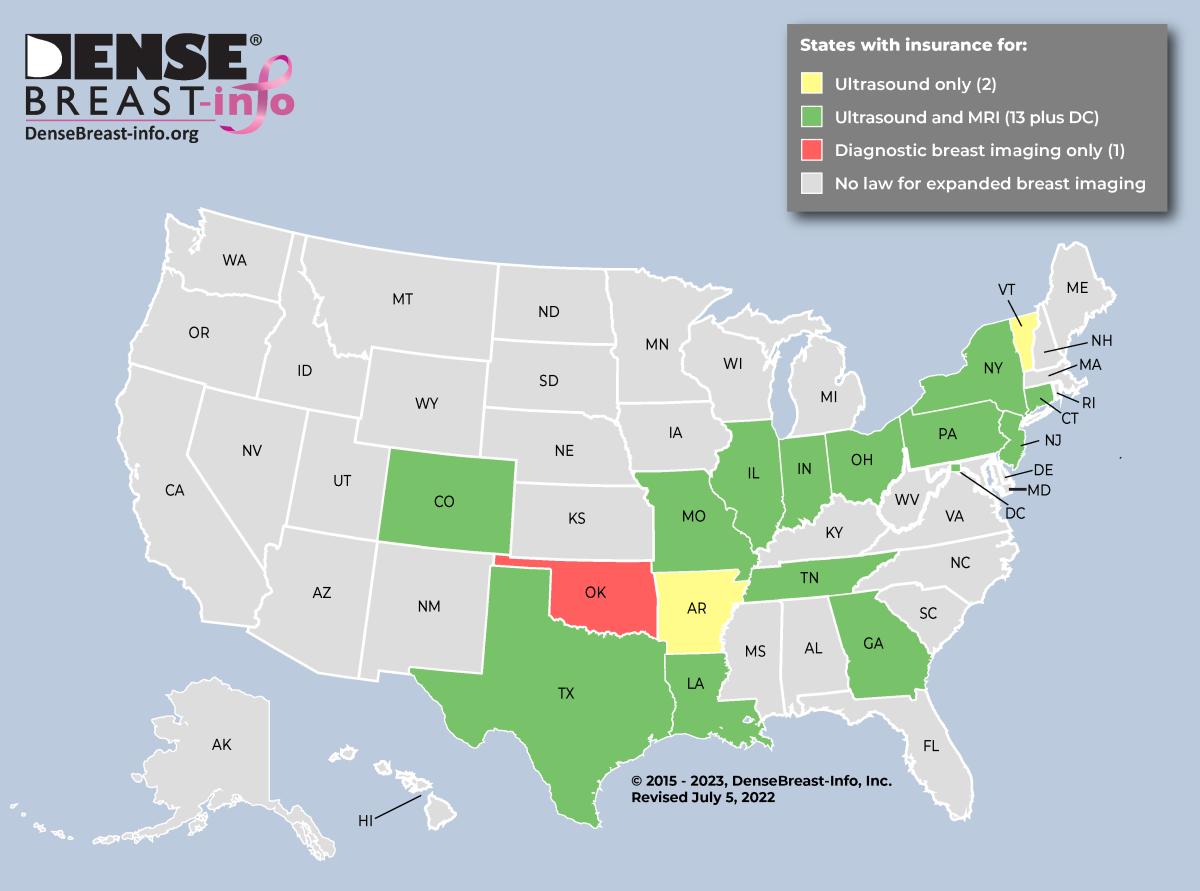
Map 1
By far the most commonly asked questions submitted to the Contact Us page on the DB-i website are from women trying to navigate insurance coverage for additional screening. It can be daunting to navigate individual insurance policy limitations and state-mandated coverage — or the lack of such mandate.
Why is This Necessary?
The vast majority of screening mammograms are performed in facilities that batch read them and where the patient has no interaction with the radiologist. Therefore, for most women, the only communication after a mammogram is their lay letter.
While my own interval cancer was growing undetected, the letters I received after my mammogram contained six words: “Normal negative, no evidence of cancer.” Not one word about my breast density or its implications.
All I was told was that my mammogram was normal. I now know it was classified as normal — not because there was no cancer and not because the radiologist knew with any certainty that there was no cancer. It was classified as normal because my breast tissue was so dense that my cancer could not be seen. My radiologist knew I had dense breasts; my referring doctor knew I had dense breasts — the only one who did not know was the one with dense breasts.
Women are often shocked to learn that in dense breasts, a mammogram reported as normal, negative or benign does not reliably mean that cancer is not present. I learned the hard way that a normal mammogram in a dense breast does NOT mean cancer is NOT growing undetected.
And what’s newsworthy about my experience is that it’s not newsworthy at all. The growing number of breast density inform laws across the nation are often created by patient advocates with similar stories.
(See Map 2.)
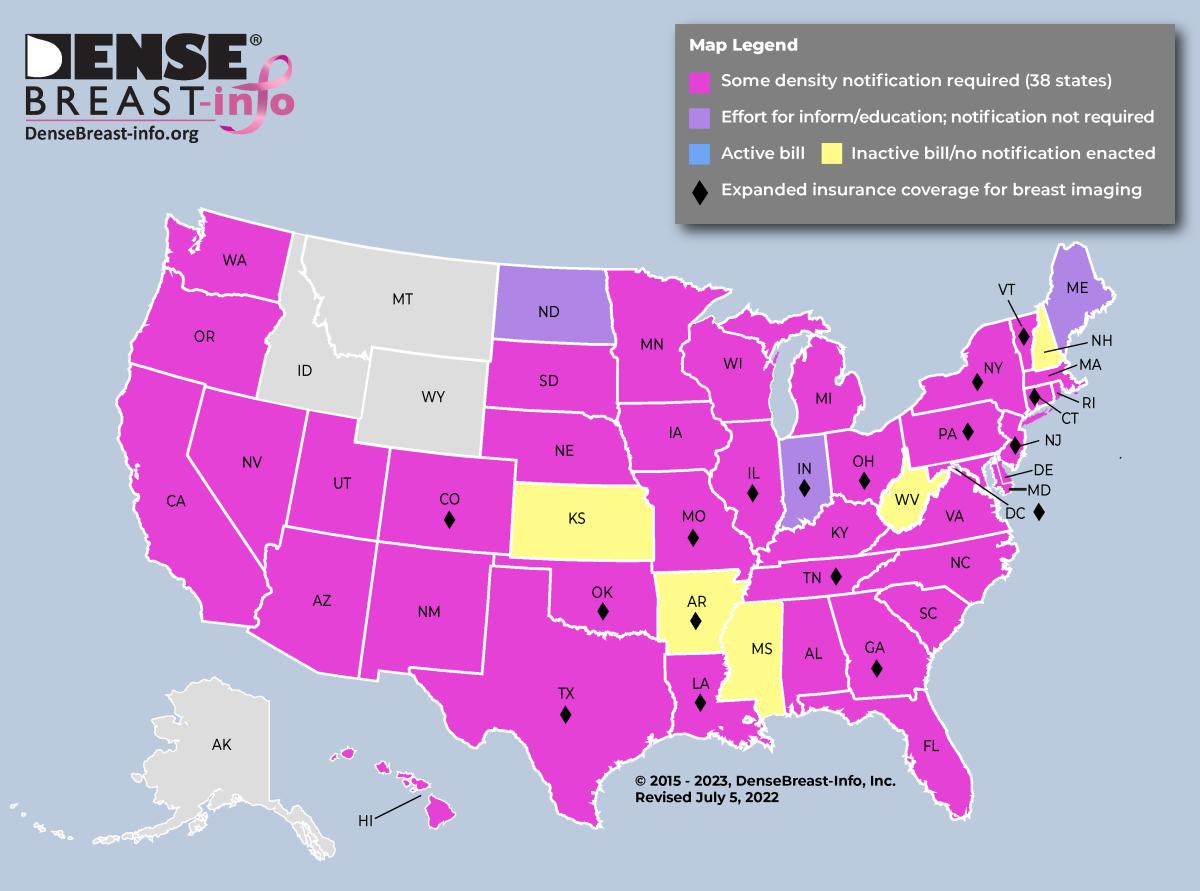
Map 2
Educating Women and Providers About Density
I’m often asked why density inform laws are necessary. Certainly, all patients should know what to expect from a mammogram, what its limitations may be, and whether a mammogram is enough. In women with extremely dense breasts, mammograms will miss about half of cancers present. Half. As a woman with extremely dense breasts, I would have valued this information and would surely have asked about additional screening. But I was denied that conversation as I was never told I had dense breasts. Patients cannot make informed choices without information.
Are Providers Ready to Have Breast Density Conversations?
We continue to focus on educating health providers about supplemental screening options and how to identify patients who may benefit from them. DB-i has published two research studies on this topic, identifying significant knowledge gaps among women’s health providers regarding breast density, breast cancer risk assessment and screening.
Results of the first study, “Radiologic Technologist and Radiologist Knowledge Gaps about Breast Density Revealed by an Online Continuing Education Course,” conducted by radiologists and radiologic technologists, were published in the Journal of Breast Imaging in July 2020. The study found important knowledge gaps regarding breast density, breast cancer risk assessment and breast cancer screening. About one-third overestimated the ability of tomosynthesis to detect cancer and thought it was similar to magnetic resonance imaging, though MRI detects another 10-16 cancers per 1,000 women that were not seen on tomosynthesis. Participants also mistakenly thought the Gail risk model should be used to determine if a woman is high risk for the purposes of recommending screening MRI or genetic testing; the Tyrer-Cuzick (IBIS) model is the most accurate model currently available and includes breast density as a risk factor.
The second study, “Effect of an educational intervention on women’s healthcare provider knowledge gaps about breast density, breast cancer risk, and screening,” was published in Menopause in April 2021. We identified important knowledge gaps about the implications of breast density among women’s healthcare providers. Further, we showed that these deficiencies can be effectively addressed with web-based education.
Our most recent study in press at the Journal of Breast Imaging is “Effect of an Educational Intervention on Women’s Health Care Provider Knowledge Gaps about Breast Cancer Risk Model Use and High-Risk Screening Recommendations.” The study details the important knowledge gaps that exist about breast cancer risk models and high-risk screening recommendations among referring women’s health care providers. Nearly all knowledge gaps were significantly reduced after web-based education.
Patient notification about breast density is likely to kick off new conversations between patients and their providers on the topic. It is important for that provider to be educated about breast density to be prepared for those conversations. This will ensure women receive appropriate breast cancer screening recommendations.
Guided by the results of these studies, DB-I launched the certified on-demand education program, Dense Breasts and Supplemental Screening. The course is now free and jointly provided by DenseBreast-info.org and the University of Pittsburgh School of Medicine and has also been accredited by the American Society for Radiologic Technologists (ASRT). Developed for primary care healthcare providers who refer women to breast screening, including family medicine, internal medicine, and OB/GYN physicians and midlevel providers, as well as radiologists and radiologic technologists, the course has been designated for 1.5 AMA PRA Category 1 credits or 1.5 ASRT Category A CE credits.
What Happens with a National Reporting Standard?
We’ve come a long way with awareness and education related to breast density — but until there is a national reporting standard and consistent insurance coverage for supplemental imaging, there is still work to do.
To that end, what happens to individual state laws if and when there is a national reporting standard? The Mammography Qualified Standards Act and Program (MQSA) allows states to set their own reporting standards as long as they are at least as “stringent” as what is required on the federal level. When a national reporting standard becomes available, each state that has a law will have to determine if their law meets the federal criteria. There are four possible scenarios that I anticipate for states based on their current density inform status:
•No current inform law: adopt federal reporting language
•Existing inform law not stringent enough: adopt federal reporting language
•Existing inform law stringent enough: maintain existing language or adopt federal reporting language
•Existing inform law with stipulation that a federal law prevails: adopt federal reporting language
The year 2023 may finally be the year that both a national reporting standard and a federal insurance mandate for no-cost additional imaging become a reality. But even if not, there is still a need for patient and provider education on the topic to ensure that women and their providers have information to make informed breast health decisions to find cancer at the earliest stage possible.

JoAnn Pushkin is executive director of DenseBreast-info, Inc. She has authored/coauthored educational courses, articles, grants, and speaks on the topic of breast density and inform efforts. For more information on breast screening, risk factors and dense breasts, visit DenseBreast-info.org.
Related Breast Density Content:
AI Provides Accurate Breast Density Classification
VIDEO: The Impact of Breast Density Technology and Legislation
VIDEO: Personalized Breast Screening and Breast Density
VIDEO: Breast Cancer Awareness - Highlights of the NCoBC 2016 Conference
Fake News: Having Dense Breast Tissue is No Big Deal
The Manic World of Social Media and Breast Cancer: Gratitude and Grief
Related Breast Imaging Content:
Single vs. Multiple Architectural Distortion on Digital Breast Tomosynthesis
Today's Mammography Advancements
Digital Breast Tomosynthesis Spot Compression Clarifies Ambiguous Findings
AI DBT Impact on Mammography Post-breast Therapy
ImageCare Centers Unveils PINK Better Mammo Service Featuring Profound AI
Radiologist Fatigue, Experience Affect Breast Imaging Call Backs
Fewer Breast Cancer Cases Between Screening Rounds with 3-D Mammography
Study Finds Racial Disparities in Access to New Mammography Technology


 March 06, 2026
March 06, 2026 









