November 10, 2021 — DenseBreast-info.org (DB-I) commemorates the 10-year anniversary of the FDA’s National Mammography Quality Assurance Advisory Committee (NMQAAC) consensus opinion that patient breast density information should be included in both patient letters and medical reports. Federal legislation, led by Sen. Dianne Feinstein (CA) and passed in 2019, requires the FDA to ensure women receive such notification. While the FDA has proposed breast density reporting language, no timing has been set for when the final ruling will be published and implemented.
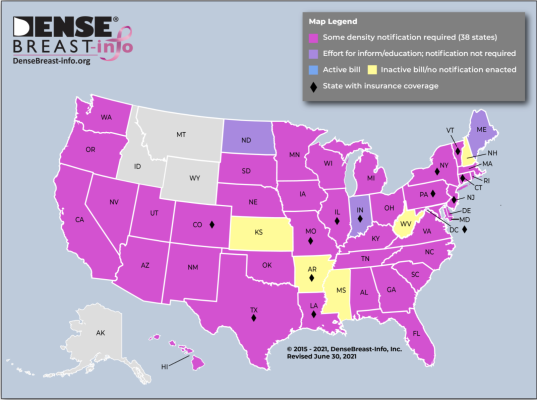
According to DB-I analysis, 38 states and DC currently require some level of density notification; however, the state laws differ significantly in depth and breadth. Six state laws only require a general notification about breast density be provided without actually telling the patient if she, herself, has dense breasts (CT, LA, MD, MO, NJ, TX). Additionally, of the 38 state laws, only about half mention that a discussion about supplemental screening is appropriate and not all mention breast density as an independent risk factor for the development of breast cancer. A single national reporting minimum would standardize information for all U.S. women.
“Every state that enacts its own ‘inform’ law adds a new version into the mix. Neighboring states may have very different notification requirements. This creates an inequity in actionable information a patient receives based on where she lives. A national reporting standard is needed to ensure consistency from state to state, promote utilization of medically-sourced educational materials, and further research on personalized screening strategies,” said JoAnn Pushkin, Executive Director of DenseBreast-info.org. Density reporting was added to the 2011 NMQAAC agenda at the written request of Pushkin, who was also invited to testify at the meeting.
According to Dr. Wendie Berg, professor of radiology, University of Pittsburgh School of Medicine, Magee-Womens Hospital, Department of Radiology and chief scientific advisor, DenseBreast-info.org, “Every woman should be informed of the possible benefits and considerations in doing a test – in this case, the realistic limitations of mammography in dense breasts. A single national reporting standard is needed which includes such key points, as:
- Unambiguous notification as to whether or not the patient herself has dense breasts
- High breast density does hide some cancers on a mammogram
- High breast density does increase the risk of developing breast cancer
- To increase cancer detection, other imaging tests, in addition to mammography, may be appropriate to consider.”
“I was proud that our bipartisan bill to ensure patients receive information about their breast density as part of their mammogram results passed in 2019. With the support of advocates and health organizations like DenseBreast-info, this legislation is now awaiting final implementation by the FDA. On the 10-year anniversary of FDA’s determination that patients should know more about breast density, I call on the agency to prioritize the release of these regulations, so all women have the information they need to make their own health care decisions,” said Sen. Feinstein.
For more information:
DenseBreast-info.org
Related Breast Density Content:
VIDEO: The Impact of Breast Density Technology and Legislation
VIDEO: Personalized Breast Screening and Breast Density
VIDEO: Breast Cancer Awareness - Highlights of the NCoBC 2016 Conference
Fake News: Having Dense Breast Tissue is No Big Deal
The Manic World of Social Media and Breast Cancer: Gratitude and Grief


 March 06, 2026
March 06, 2026 









