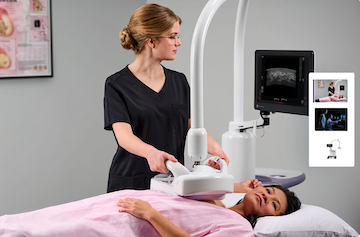
March 20, 2025 — GE HealthCare has launched Invenia Automated Breast Ultrasound (ABUS) Premium, the latest 3D ultrasound offering advanced artificial intelligence (AI) and innovative features to drive faster*, reproducible supplemental screening and streamline exam readings on patients with dense breasts.
Approximately 71% of cancers occur in dense breasts.[1] According to studies across the U.S. and Europe, 40% of women[2] and 70% of Asian women[3] have dense breast tissue, making them four to six times more likely to receive a breast cancer diagnosis.[4] Early detection of breast cancer is critical, yet about one-third[5] of cancers in dense breasts may go undetected by a mammogram, as the cancer can be masked within the dense tissue. There is rapidly growing evidence supporting ABUS as a valuable tool for detecting cancer in dense breasts because it provides clearer and more detailed images and has been shown to improve the sensitivity of detecting invasive cancer in dense breasts when added to mammography.[6],[7],[8],[9]
“Women with dense breasts often face poorer outcomes due to malignancies detected at later, more advanced stages. Invenia ABUS Premium equipped with AI has the potential to optimize clinicians’ screening capabilities, enabling them to detect even small, early-stage cancers with a high degree of confidence in women with dense breasts,” said Karley Yoder, CEO of Comprehensive Care Ultrasound, GE HealthCare. “Invenia ABUS Premium is designed to help deliver the best possible outcomes for patients while also prioritizing the patient experience with features to improve scan speed and enhance comfort during an exam.”
Invenia ABUS Premium is designed to manage high patient volumes along the breast care pathway, while delivering extraordinary image quality and boosting clinical confidence. The new Verisound AI tools can help clinicians work smarter and more efficiently, featuring Scan Quality Assessment for immediate qualitative evaluation during the exam for proper breast coverage and positioning, and Auto Nipple Detection for consistent nipple marker positions. The new Fast Scan tool increases scan speed by up to 40%*, while cSound Imageformer capabilities automatically create focus at every pixel, ensuring consistent, high-resolution image quality and reproducibility.
For fast, streamlined reading, physicians can use Invenia ABUS Viewer with AI Assistant** to quickly review and interpret patient exams either from their practice or remotely. The integration of AI tools** for enhanced review of ABUS 3D datasets harness intelligent algorithms to assist in detecting and characterizing breast lesions. These tools can help improve clinical confidence and reading efficiency, addressing staff shortages.
The ABUS Premium’s non-invasive design significantly enhances the patient experience, with a patient experience study showing that 100% of women would recommend an ABUS exam to their best friend.[10] This advanced ABUS technology also has the potential to reduce unnecessary biopsies by providing specific image features to distinguish and detect malignant tumors, enabling clinicians to escalate care sooner.[9] Additionally, it reduces patient exposure to radiation or contrast injection as it doesn't use any iodinated contrast agent or ionizing radiation. The new Reverse Curve™ transducer follows the contour of a woman’s breast anatomy, while selectable compression levels provide personalized comfort during the exam.
"As an early adopter of ABUS, I've experienced the entire evolution of this technology. The Invenia ABUS Premium is superior* with its special AI features, which ensure the breast volume is captured and offer automatic nipple annotation, reducing scanning time and streamlining clinical workflow," said Athina Vourtsis, MD, PhD, Chief Director and Founder of Athena Medical, Athens, Greece; Founding President of the Hellenic Breast Imaging Society; European Liaison and Member of the Medical Advisory Board of DenseBreast-Info. "The new Reverse Curve transducer makes the examination more patient friendly, and it is much more comfortable. There has been great improvement in image quality with less shadowing, and the structures behind the area of the nipple are seen more clearly, providing radiologists with greater diagnostic confidence."
Invenia ABUS Premium will be launching in key countries throughout 2025 and received Premarket Approval (PMA) from the U.S. Food and Drug Administration. Invenia ABUS Premium was featured at the 2025 European Congress of Radiology (ECR) and will also be showcased at the National Consortium of Breast Centers (NCoBC) 2025 and 2025 Society of Breast Imaging (SBI) Symposium.
Learn more about GE HealthCare’s Invenia ABUS Premium here: Invenia ABUS Breast Imaging Ultrasound | GE HealthCare (United States)
*Compared to InveniaTM ABUS 2.0.
**AI Assistant available third-party tools include QVCAD, Koios DS Breast, BU-CAD and MONCAD ABS. Not available in all regions.
[1] Arora N, King TA, Jacks LM., Ann Surg Onc, 2010; 17:S211-18.
[2] Pisano et al. NEJM 2005; 353: 1773.
[3] Ref. Ellison-Loschman, et al, PLOS ONE July 2013.
[4] Boyd NF et al. NEJM 2007; 356: 227
[5] Mandelson et al. J Natl Cancer Inst 2000; 92:1081-1087.
[6] FDA PMA P110006 summary of safety and effectiveness
[7] Brem RF, Tabár L, et.al. Radiology. 2015 Mar; 274(3): 663-73.
[8] Stempel et.al., Journal of Breast Imaging 2024, Vol XX, No. XX, 1-10.
[9] Wenhui Ren et. al, Elsevier Acad Radiol 2023; 30:S114–S126.
[10] Shah et.al. Journal of Diagnostic Medical Sonography DOI: 10.1177/8756479313476920 2013.


 March 06, 2026
March 06, 2026 









