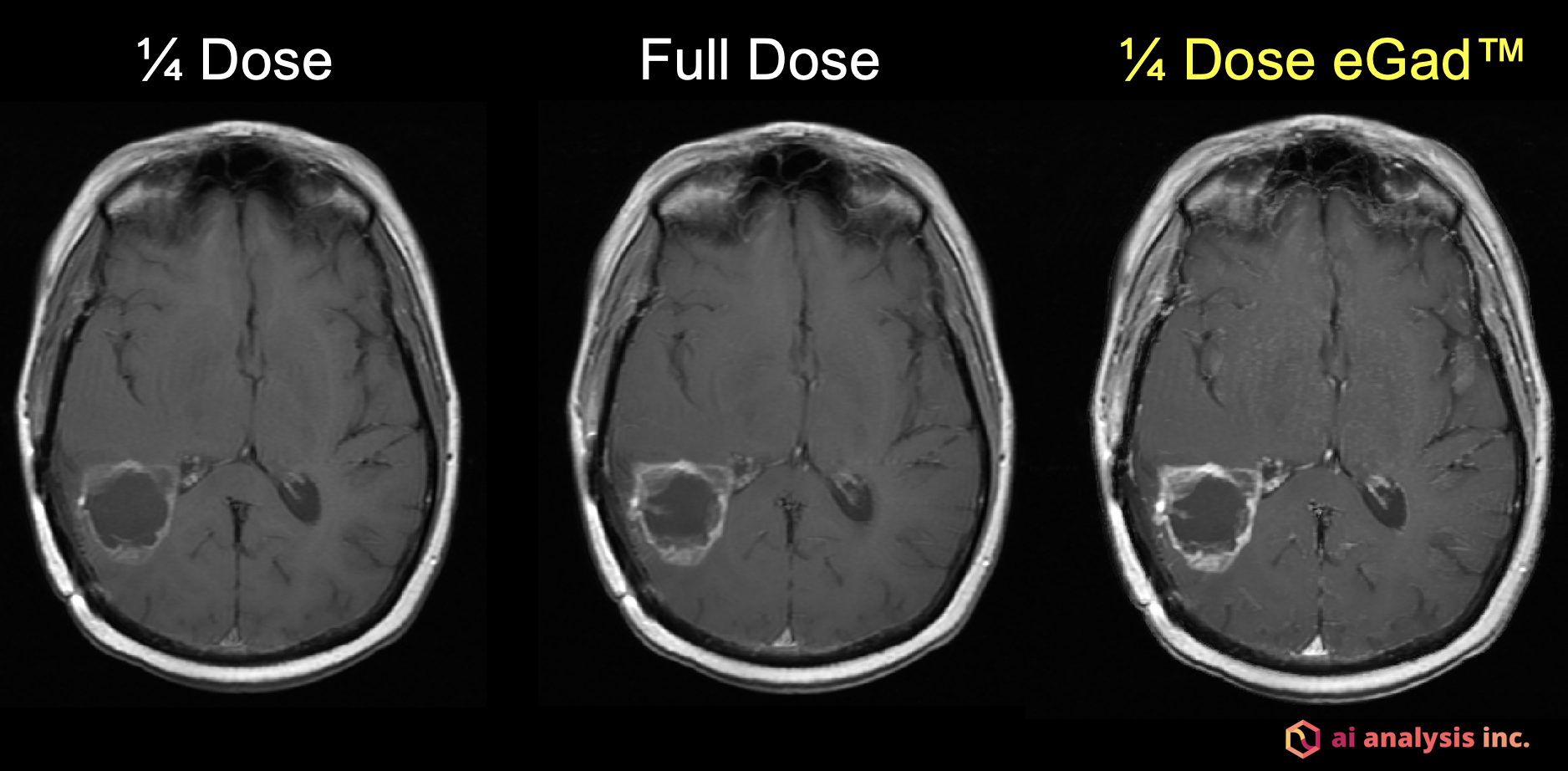
This image is of an 80 kg woman with a newly diagnosed IDH-wildtype glioblastoma. The quarter dose image on the left was obtained after the administration of 4 ml of MultiHance. Subsequently, an additional 12 ml of MultiHance was administered and the cumulative full dose image in the center was obtained. The image on the right was rendered following artificial intelligence processing of the 4 ml image using eGad genetic algorithms. This image has the quality of triple dose gadolinium even though only one quarter dose gadolinium was given.
Gadolinium-based contrast agents (GBCAs) are widely used in medical imaging to provide essential diagnostic information from magnetic resonance imaging (MRI) scans. These injections help a radiologist examine images more accurately by improving the visibility and the clarity of many abnormalities and for achieving maximum diagnostic quality.
However, recent research has shown that gadolinium agents may remain in the body — including the liver, bone and brain — for months or even years after injection.1 This has stoked fears of adverse clinical outcomes from the use of GBCAs. It remains unclear if there are any neurological signs or symptoms associated with gadolinium retention although it must be considered that up to now, no negative health effects can be attributed to gadolinium retention in the brain.2,3
Despite agreement among the American College of Radiology (ACR) and the U.S. Food and Drug Administration (FDA) that gadolinium retention is not known to have adverse outcomes in patients,2,3 some practitioners still have concerns about the use of GBCAs. Would those concerns be alleviated if lower levels of the safest GBCAs could be used?
Using Algorithms to Test GBCAs
To address this question, A. I. Analysis, Inc., a Seattle-based medical software company, designed genetic algorithms that allow for the use of lower dose GBCAs. When low dose gadolinium was injected into patients and the resultant images were processed using these artificial intelligence (AI) techniques (eGad), the resultant images all equaled or exceeded the quality of full contrast dose images. Additionally, A. I. Analysis, Inc. used its advanced, layered machine learning system tools to verify that equimolar doses of the standard concentration gadoteridol (ProHance), a macrocyclic contrast agent which has been shown from animal studies to result in less retention one month after injection compared to other agents,4,5 is non-inferior to the more concentrated gadolinium agent gadobutrol (Gadavist).6
In the GBCA comparison study, 32 patients with glioblastoma (GBM) received both ProHance and Gadavist using double-blind, randomized crossover methodology. To evaluate the images, the authors used the Quantitative Enhancement Analysis software of A.I. Analysis, Inc. The software identified no statistically significant difference in enhancement characteristics between the standard concentration of ProHance and the double concentration of Gadavist when administered at the same equimolar dose of 0.1 mmol/kg. This confirmed the findings of the original TRUTH study,7 a landmark gadolinium contrast agent comparison study from 2015, which found no benefit to the use of higher concentrations of gadolinium using traditional comparison techniques.
Additionally, 50 patients with known contrast enhancing brain tumors initially received either 1/10, 1/4, 1/3 or 1/2 dose of a high relaxivity GBCA (gadobenate dimeglumine, MultiHance) and routine images were acquired. Subsequently, the remaining cumulative full dose images were acquired. The original low dose images were processed using the eGad synthetic enhancement tool of A.I. Analysis, Inc., and three blinded readers confirmed that the low dose eGad images achieved image quality equal or greater than the cumulative full dose images.
Using AI Applications
In light of concerns about gadolinium retention, these findings will help radiologists make the best decisions with and for their patients with confidence. The use of AI tools for future studies will allow for more sophisticated research and improved results.
As we enter 2020, the use of AI applications to compare agents and medical images will become more common. As these studies demonstrate, the use of AI in research can rapidly produce findings that are both transparent and replicable, the hallmarks of scientific validity.
There is more in a medical image than the human eye can understand, and AI can help us make better decisions and better treatment plans for our patients.
Key results described here were presented at the 2019 Radiological Society of North America (RSNA) meeting.
Matthew Kuhn, M.D., FACR, is board certified in neuroradiology and diagnostic radiology and holds certificates of proficiency in coronary CT angiography and cardiac and peripheral vascular MRI from the American College of Radiology. Kuhn is a clinical professor at the University of Illinois College of Medicine in Peoria, Ill. and is a practicing radiologist with Radiology Partners.
Disclosure: Matthew Kuhn, M.D., FACR serves as chief medical officer for A.I. Analysis, Inc., a company that has its roots in fully automated and reproducible change detection, and its eyes on a future in which AI broadly enhances radiologist performance and patient well-being, by turning information overload into information affluence.
References:
1. McDonald RJ, et al. Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates. Radiology 289(2): 517-534 2018
2. ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. Version 10.3.2018. Accessed January 2019.
3. FDA Drug Safety Communication: New warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. FDA website. https://www.fda.gov/Drugs/DrugSafety/ucm223966.htm. Accessed December 19, 2018.
4. Bussi S, et al. Differences in Gadolinium Retention After Repeated Injections of Macrocyclic MR Contrast Agents to Rats. J Magn Reson Imaging. 2018; 47:746-752
5. McDonald RJ, et al. Comparison of Gadolinium Concentrations within Multiple Rat Organs after Intravenous Administration of Linear Versus Macrocyclic Gadolinium Chelates. Radiology 285:2: 2017
6. Kuhn MJ, Patriarche JW. The Use of Artificial Intelligence to Evaluate Differences in Contrast Enhancement between Two Macrocyclic Gadolinium Agents in Patients with Glioblastoma Multiforme [abstract]. In: Proceedings of the 105th Annual Meeting of the Radiological Society of North America. 2019 Dec 1–6; Chicago, IL. Abstract nr SSM21-04.
7. Maravilla KR et al. Are there differences between macrocyclic gadolinium contrast agents for brain tumor imaging? Results of a multicenter intraindividual crossover comparison of gadobutrol with gadoteridol (the TRUTH study). AJNR Am J Neuroradiol. 2015;36(1):14-23.
Related Content of MRI Gadolinium Safety Concerns
The Debate Over Gadolinium MRI Contrast Toxicity
VIDEO: How Serious is MRI Gadolinium Retention in the Brain and Body? An interview with Max Wintermark, M.D.
VIDEO “Big Concerns Remain for MRI Gadolinium Contrast Safety at RSNA 2017,” An interview with Emanuel Kanal, M.D.
Radiology Has Failed to Properly Assess or Track MRI Gadolinium Contrast Safety
Recent Developments in Contrast Media
FDA Committee Votes to Expand Warning Labels on Gadolinium-Based Contrast Agents
European Medicines Agency Issues Update on Gadolinium Contrast Agents
ISMRM Issues Guidelines for MRI Gadolinium Contrast Agents
FDA: No Harm in MRI Gadolinium Retention in the Brain
VIDEO: MRI Gadolinium Contrast Retention in the Brain
Gadolinium May Remain in Brain After Contrast MRI



 March 10, 2026
March 10, 2026 









