July 11, 2014 — The Texas Center for Proton Therapy received its new cyclotron this month, and the center is expected to treat its first patients in early 2016. The center is a collaboration of Texas Oncology, the US Oncology Network and McKesson Specialty Health.
July 11, 2014 — The American Society for Radiation Oncology (ASTRO) and the American Association of Physicists in Medicine (AAPM) announced the launch of RO-ILS, or Radiation Oncology Incident Learning System, a new, national patient safety initiative to facilitate safer and higher quality radiation oncology care.
Biotronik announced that the first device patients to undergo full-body magnetic resonance imaging (MRI) scans in the United States have been implanted with the Biotronik DX System.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
This August marks the 100th anniversary of the start of World War I in 1914. A few months after the start of the war, in early 1915, the Germans began using Zeppelins in the first application of strategic bombing over British cities. Zeppelin raids continued over Russia, Belgium, France, Italy and Britain for the duration of the war. These raids ironically paved the way for U.S. government policy that guaranteed access to the cryogenic gas required to operate today’s magnetic resonance imaging (MRI) systems.
July 8, 2014 — A brain imaging study shows that patients with chronic fatigue syndrome may have reduced responses, compared with healthy controls, in a region of the brain connected with fatigue. The findings suggest that chronic fatigue syndrome (CFS) is associated with changes in the brain involving brain circuits that regulate motor activity and motivation. The results were scheduled for publication in the journal PLOS One.
July 8, 2014 — NorthStar Medical Radioisotopes LLC has signed a pledge with the Preparatory Commission for the Comprehensive Nuclear-Test-Ban Treaty Organization (CTBTO), designed to help the CTBTO detect nuclear testing.
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
July 8, 2014 — Humana Inc. announced earlier this year an expanded coverage policy for cardiac CT (computed tomography). Many of the new Humana indications have long been advocated for by the Society of Cardiovascular Computed Tomography (SCCT) and described in the model local coverage determination policy (LCD) developed by the organization.
NDS Surgical Imaging (NDSsi) has released its new Dome S6c widescreen color 6MP diagnostic display, now in production.
Barco announced the launch of Eonis 21” — a new 21-inch clinical review display offering a 40 percent higher calibrated luminance and almost twice the contrast ratio of the previous generation.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Canon U.S.A. Inc. and wholly owned subsidiary Virtual Imaging Inc. announced the introduction of the RadPRO Omnera 400A auto-positioning digital radiographic system, the first of a new series of overhead radiographic systems designed to help healthcare professionals meet the challenges of demanding hospital imaging departments.
The Idaho Supreme Court affirmed the October 2011 jury verdict in MRI Associates’ $52 million lawsuit against Saint Alphonsus Regional Medical Center. The ruling is the latest, and last, in a landmark legal battle between the two parties that began in 2004.
Vital Images Inc. announced the global release of VitreaExtend, an advanced visualization solution that supports up to three simultaneous users with high performance similar to Vital's advanced server-based solution.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
The ability to generate patient specific mammographic dose using volumetric breast density data from 2-D mammography and 3-D tomosynthesis was among the research presented at the 12th International Workshop on Breast Imaging (IWDM 2014), June 29-July 2, 2014.
Elekta has selected Birst, the global leader in cloud-based business intelligence (BI), to help clinicians, researchers and business managers exploit the potential of big data in their decision-making.
Delegates from academia and industry flocked to see the world's first commercial, cryogen free, 7.0T, preclinical magnetic resonance imaging (MRI) imaging, which went on display for the first time at the recent joint ISMRM/ESMRMB meeting in Milan, Italy and the SNMMI meeting in St Louis.
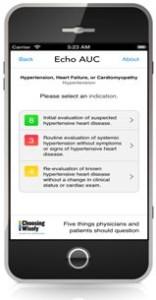
The American Society of Echocardiography Foundation (ASEF) launched the second version of its free mobile Echo AUC app at the ASE Silver Anniversary Scientific Sessions in Portland, Ore., last week.
There were several new cardiovascular ultrasound technology advances highlighted at the American Society of Echocardiography (ASE) annual meeting June 20-24 in Portland, Ore. Among these was the first commercial software released to analyze vector flow imaging, a possible new way to help with early diagnoses of some types of cardiovascular disease prior to symptoms developing. Another technology of note was high-resolution 3-D reconstructions of vascular plaques and any associated angiogenesis vasa vasorum, which could be a new way to noninvasively identify vulnerable plaque.
More than 55 companies displayed their latest products and services at the American Society of Echocardiography (ASE) 25th Annual Scientific Sessions June 20-24 in Portland, Ore. The meeting is the largest for cardiovascular ultrasound. Highlights from the show floor included:
April 8 has come and gone. Microsoft XP support is over. Our use of XP-based picture archive and communications system (PACS) should be as well. But it’s not.
Along with the growing amount of data being collected in electronic medical records (EMR) and other information repositories is the growing need to dissect and analyze that data fast and efficiently. With hospitals and practices looking to maximize efficiencies and optimize quality within their operations, having a set of analytics tools can be a key strategy for moving forward with an evolving healthcare environment.


 July 11, 2014
July 11, 2014