October 28, 2019 — Office based labs (OBLs) and ambulatory surgery centers (ASCs)…
Technology
Search by Company Name, Product Category, Product Name or by any combination of the three using the search boxes below.
Exact Imaging received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Sterile…
Advanced X-ray imaging systems developer Turner Imaging Systems announced the company has…
Canon Medical Systems USA Inc. has received U.S. Food and Drug Administration (FDA) 510(k)…
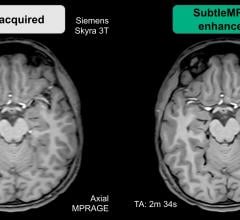
Subtle Medical announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) to…
NeuroLogica, a subsidiary of Samsung Electronics Co. Ltd., announced that the Samsung Hera I10…
The U.S. Food and Drug Administration (FDA) has cleared three modules of AI-Rad Companion Chest…
The U.S. Food and Drug Administration (FDA) has cleared the Artis icono, a high-precision family…
The Akesis Galaxy, a gamma stereotactic radiosurgery system (SRS) with continuous 360-degree…
3D Systems has received additional U.S. Food and Drug Administration (FDA) 510(k) clearance for…
IBA Dosimetry GmbH announced the launch of the new enhanced myQA Machines 2019 software…
GE Healthcare announced the U.S. Food and Drug Administration’s (FDA) 510(k) clearance of…
Bayer announced the introduction of the Medrad Stellant Flex computed tomography (CT) injection…
LAP LLC announced its Apollo MR3T positioning laser for radiation therapy has received U.S. Food…
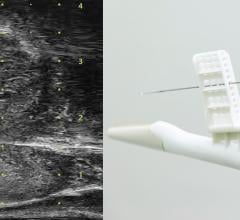
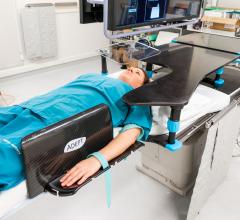
New Zealand-based Adept Medical announced the launch of the Antegrade IR Platform. Clinically…
August 30, 2019 — The CIRS Ultrasound…
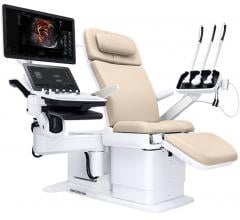
Profound Medical Corp. announced it has received 510(k) clearance from the U.S. Food and Drug…
August 14, 2019 — NinePoint Medical Inc. announced it has received U.S.
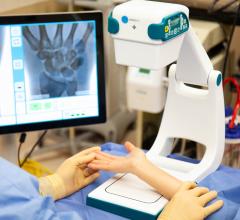
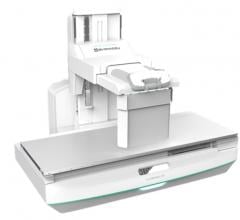
Shimadzu Medical Systems USA, a subsidiary of Shimadzu Corp., announced they have received U.S.…
Synaptive Medical announced the U.S. launch and availability of Modus Plan featuring…


 October 28, 2019
October 28, 2019