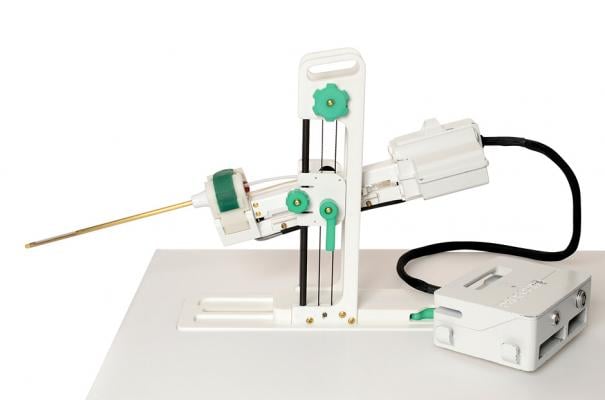
August 16, 2019 — Profound Medical Corp. announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market Tulsa-Pro for ablation of prostate tissue.
Tulsa-Pro is a transurethral prostate tissue ablation system that combines real-time magnetic resonance imaging (MRI) with robotically-driven directional thermal ultrasound and closed-loop temperature feedback control software to deliver predictable physician prescribed ablation of whole-gland or partial prostate tissue. The Tulsa-Pro system is designed to provide customizable and predictable, incision-free and radiation-free prostate ablation while actively protecting the urethra and rectum with water cooling to preserve men’s functional abilities.
The FDA’s clearance of Tulsa-Pro was based on the company’s TACT pivotal clinical trial, which met all of its primary and secondary efficacy and safety endpoints. TACT enrolled 115 patients across the United States, Canada and Europe with biopsy-proven, organ-confined prostate cancer (67 percent and 33 percent of subjects had NCCN intermediate- and low-risk disease, respectively). All patients received primary treatment of whole-gland prostate ablation with sparing of the urethra and urinary sphincter. TACT demonstrated that the Tulsa-Pro provides safe and effective prostate tissue ablation, with minimal adverse events, significant prostate volume and prostate specific antigen (PSA) reduction, and low rates of residual prostate disease. The favorable safety profile offered by the Tulsa-Pro contrasts with radical prostatectomy and radiation therapy that can leave many men with permanent erectile dysfunction, urinary incontinence and bowel dysfunction. The TACT study also demonstrated a favorable risk-benefit profile in the context of other ablative approaches, including whole-gland high intensity focused ultrasound (HIFU) and cryotherapy.
The FDA label for Tulsa-Pro will allow U.S. surgeons to perform prostate tissue ablation procedures indiscriminate of tissue type, including malignant and benign.
“We are pleased with the FDA’s expeditious review of our application,” said Arun Menawat, Profound’s CEO. “We believe this, combined with the label that the Agency approved for TULSA-PRO®, serves as a testament to our technology’s strong clinical profile.”
Profound Medical CEO Arun Menawat said the company is preparing for the U.S. commercial launch of Tulsa-Pro in the fourth quarter of 2019.
For more information: www.profoundmedical.com
Related Content
Profound Medical Corp. Announces First Paid Procedure Using TULSA-PRO System
Profound Medical Corp. and Philips Announce First TULSA-PRO Sale in Finland


 February 13, 2026
February 13, 2026 









