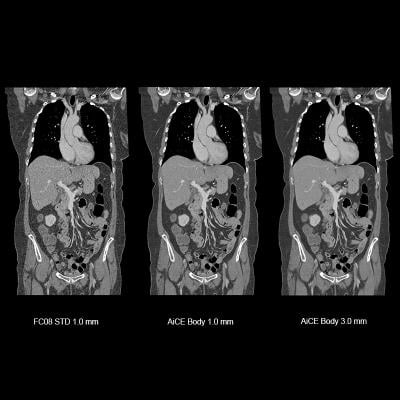
Image courtesy of Canon Medical Systems
October 21, 2019 — Canon Medical Systems USA Inc. has received U.S. Food and Drug Administration (FDA) 510(k) clearance on its Advanced Intelligent Clear-IQ Engine (AiCE) for the Aquilion Precision computed tomography (CT) system. The clearance further expands access to Canon’s new deep convolutional neural network (DCNN) image reconstruction technology. This technology, now available on both the Aquilion Precision and Aquilion One/Genesis Edition premium CT systems, uses a deep learning algorithm to differentiate signal from noise so that it can suppress noise while enhancing signal, forging a new frontier for CT image reconstruction.
Aquilion Precision, which Canon calls the world’s first ultra-high resolution CT, provides two times the resolution of conventional CT, revealing detail that is typically only seen in cardiac catheterization labs. With AiCE, the system now enables clinicians to perform super-high resolution studies at doses equivalent to standard resolution CT (with traditional hybrid iterative reconstruction techniques). AiCE learns from the high image quality of model-based iterative reconstruction (MBIR) to reconstruct CT images with improved high contrast spatial resolution.
For more information: www.us.medical.canon
Related Content
Canon Medical Receives FDA Clearance for AiCE Reconstruction Technology for CT
Canon Medical Systems' Aquilion Precision CT Receives FDA Clearance


 March 09, 2026
March 09, 2026 









