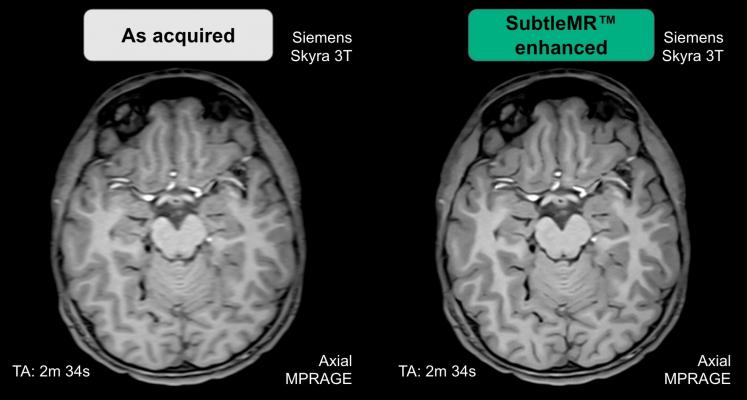
October 16, 2019 — Subtle Medical announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market SubtleMR. SubtleMR is an image processing software that uses denoising and resolution enhancement to improve image quality for magnetic resonance imaging (MRI) scans.
SubtleMR utilizes proprietary deep learning algorithms to bring the latest imaging enhancement technology to existing scanners. It is currently in pilot clinical use in multiple university hospitals and imaging centers.
"One of the most exciting things about deep learning reconstruction is how it redefines the usual negotiation between exam time and image quality. This could lead to significant downstream value for imaging operations and for patient experience," said Christopher Hess, M.D., chair of the Department of Radiology and Biomedical Imaging at UCSF.
Read the article “Using Artificial Intelligence to Reduce Gadolinium Contrast”
SubtleMR delivers a significant improvement in the quality of noisy images, which is particularly beneficial for patients who have difficulty holding still for long periods of time. Artifact-ridden images and the need for re-scans are a challenge for both patients and physicians. SubtleMR integrates seamlessly into the radiology workflow, and it is compatible with any brand of MRI scanner and picture archiving and communication system (PACS).
Subtle Medical has the first and only artificial intelligence (AI) solutions, according to the company, to receive FDA clearance for medical imaging enhancement — SubtlePETand SubtleMR. The company's first product, SubtlePET, received FDA clearance in 2018 for the enhancement of images from up to four times faster positron emission tomography (PET) scans. The company's third product in development, SubtleGAD, has the potential to reduce gadolinium dosage during contrast-enhanced MRI exams. The company was recently awarded a $1.6 million NIH grant to further its research on this technology.
Watch the VIDEO: Artificial Intelligence May Help Reduce Gadolinium Dose in MRI
For more information: www.subtlemedical.com


 February 05, 2026
February 05, 2026 









