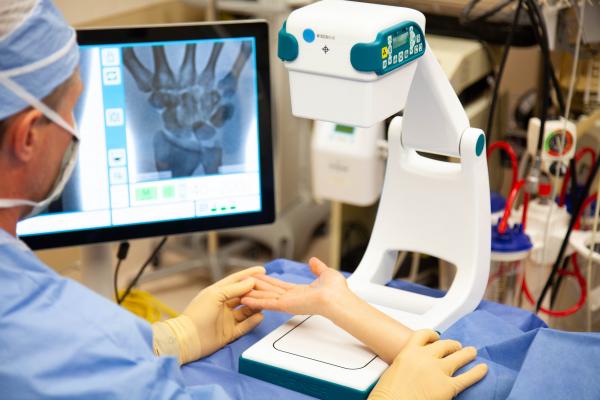
October 23, 2019 — Advanced X-ray imaging systems developer Turner Imaging Systems announced the company has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Smart-C mini C-arm portable fluoroscopy and X-ray imaging device. Focused on mobility and portability, the patent-pending Smart-C weighs only 16 pounds so that it can be hand-carried to the point of care, with no need for special equipment to wheel the device around.
Turner Imaging Systems will launch the Smart-C into U.S. markets, followed quickly worldwide. The company will showcase the Smart-C at MEDICA, the world’s largest medical exhibition, Nov. 18-21 in Dusseldorf, Germany, followed by the Radiological Society of North America (RSNA) annual meeting, Dec. 1-6 in Chicago.
Turner Imaging Systems Founder and CEO D. Clark Turner, Ph.D., said the company envisions the Smart-C being particularly useful for humanitarian aid workers, including groups such as Doctors Without Borders. Other specialty applications he highlighted include imaging in sports medicine, especially on the field or in the locker room, in-office outpatient orthopedic surgeries, care on military battlefields, extremity injections for pain management, mobile radiology units in rural areas, emergency rooms and more.
The Smart-C is the first device in a pipeline of products that will employ similar technologies as the Smart-C.
Howard Berger, M.D., president and CEO of RadNet Inc. commented, “As one of the largest purchasers and evaluators of imaging equipment in the U.S., I believe Smart-C is one of the most unique innovations to be introduced to our industry in many years. Its portability, cordless design and high-quality images will attract users in multiple diagnostic imaging settings. As RadNet was an early investor in Turner Imaging Systems, we congratulate the company’s management and employee base in receiving FDA clearance.”
For more information: www.turnerxray.com

 March 05, 2026
March 05, 2026