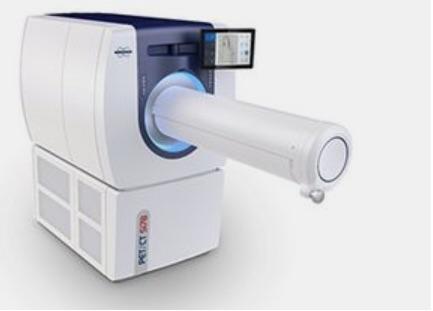
September 26, 2018 — Bruker recently announced the introduction of the new preclinical PET/CT Si78 scanner for whole-body molecular imaging at the World Molecular Imaging Congress, Sept. 12-15 in Seattle. It combines Bruker's novel positron emission tomography (PET) and micro-computed tomography (CT) technologies in a single scanner operated by the multimodality imaging software platform ParaVision 360.
The PET/CT Si78 addresses the latest needs of preclinical imaging scientists by combining homogeneous sub-millimeter PET spatial resolution over a large field of view (FoV) with minimal X-ray radiation dose. The whole-body micro-CT scan times can be shorter than 7 seconds, which is important for animal welfare.
The PET/CT Si78 next-generation nuclear molecular imaging (NMI) system combines Bruker's 'total body' PET detector, which provides sub-millimeter spatial resolution over a large field-of-view and high PET sensitivity with a very fast, low dose and high-resolution micro-CT. The ParaVision 360 software seamlessly integrates PET/CT workflows from animal positioning and imaging to data processing and management. This approach supports reproducibility and consistent PET quantification to facilitate global preclinical discovery, development and validation processes.
For more information: www.bruker.com


 February 13, 2026
February 13, 2026 









