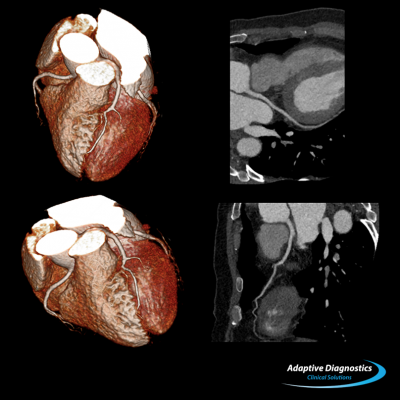
Cardiac CT image of a patient with atrial fibrillation using the Sure Cardio prospective arrhythmia detection software.
- SureSubtraction, which has the ability to remove bone and calcium from data sets while allowing clinicians to view tumors or arteries at risk. SureSubtraction is available for brain, bone, carotid and coronary artery exams.
- Metal Artifact Reduction (MAR), which removes streak artifacts in images due to metallic implants in the body.
- Sure Cardio: Prospective with arrhythmia detection is a unique application that dramatically lowers patient dose during coronary CTA exams using a helical acquisition technique to provide one continuous image instead of multiple images. It automatically detects and adjusts to patients with irregular heartbeats, providing quicker, more conclusive exam results.
- Variable Helical Pitch (vHP), which has the ability to automatically change an electrocardiogram (ECG)-gated to a non-ECG-gated acquisition, reducing IV contrast and radiation dose. vHP can significantly reduce radiation dose over the use of a single-gated pitch setting.
- Dual Energy, which uses two energies during one CT scan, providing clinicians with more data to help quantify and characterize anatomy and lesions. As a result, exam times and radiation dose are both reduced.


 February 09, 2026
February 09, 2026 









