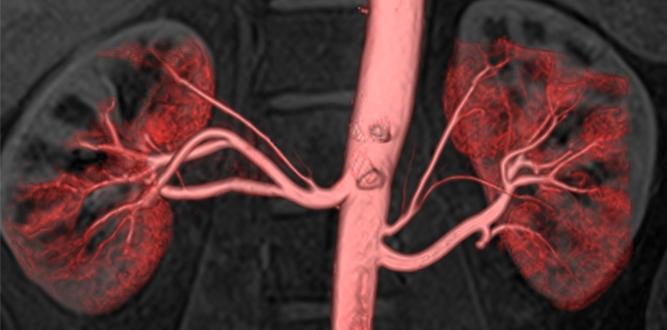
Image courtesy of Toshiba
January 16, 2015 — Non-contrast enhanced MR angiography (MRA) techniques have attracted interest in the medical community for diagnosing renal artery stenosis, as it eliminates the need for contrast agents used with computed tomography angiography (CTA). In an international multi-center trial REACT (REnal Artery Contrast-free Trial), Toshiba’s Time-Spatial Labeling Inversion Pulse (Time-SLIP) is validated as a technique for non-contrast vascular imaging while providing an accurate and non-invasive method for diagnosing patients.
The results from the multi-center trial showed no statistical differences between non-contrast MRA and CTA in terms of their ability to visualize renal artery stenosis. The results from the trial were published in the January 2015 issue of American Journal of Roentgenology.
Toshio Takiguchi, president, Toshiba Medical Systems Corporation (TMSC) comments: “The REACT study has proved that it is now possible to detect renal artery stenosis without using contrast agents. Time-SLIP eliminates the need for gadolinium-based contrast agents, which results in safer and more comfortable examinations. Not only do these non-invasive and painless techniques eliminate the risks associated with gadolinium-based contrast agents, but they also require less setup time for clinicians, resulting in MR exams that are more comfortable for the patient and can be completed more quickly without compromising image quality.”
The trial consisted of 75 patients in collaboration with seven medical centers in five countries: the United States, Spain, China, France and Japan. All subjects underwent medically required non-contrast renal MRA exams, using the 1.5T Vantage Atlas and Vantage Titan MR systems, as well as CT angiography for the evaluation of renal artery stenosis.
For more information: www.medical.toshiba.com


 February 13, 2026
February 13, 2026 









