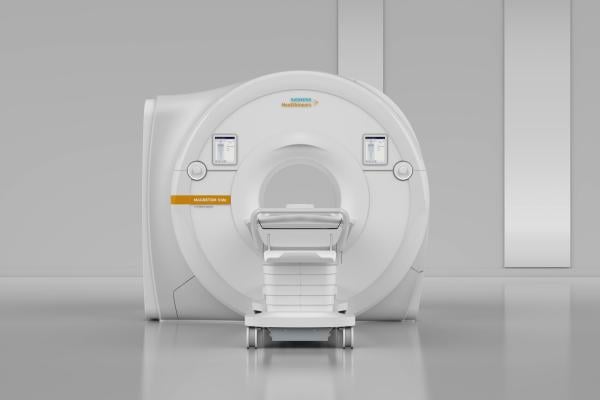
November 2, 2017 — Mallinckrodt Institute of Radiology at Washington University School of Medicine, St. Louis, recently became the first U.S. healthcare institution to install the Magnetom Vida 3 Tesla (3T) magnetic resonance imaging (MRI) scanner from Siemens Healthineers. The scanner features new BioMatrix technology that addresses patients’ anatomical and physiological differences as well as user variability.
The 70-cm scanner includes a new magnet design and up to 60/200 XT gradient performance to support high-end clinical applications. Its BioMatrix technology — a collection of sensors, tuners and interfaces — enables the scanner to adapt automatically to anatomical and physiological characteristics to provide consistent, high-quality imaging for all patient types. The scanner’s architecture and innovative applications simplify and accelerate workflows while increasing exam precision and patient comfort. Its GO technologies automate and simplify workflows from the start of the scan through quality control of the image data, resulting in increased productivity for routine examinations throughout the body. And the Eco-Power technology deactivates power-hungry components that are inactive for long periods, potentially lowering the total cost of ownership over the scanner’s lifetime.
“We’re using the scanner in clinical trials and other research studies involving cancer patients and patients with neurodegenerative diseases, such as Alzheimer’s, as well as for cardiac imaging,” said Pamela K. Woodard, M.D., senior vice chair and division director of radiology research facilities at Washington University’s Mallinckrodt Institute of Radiology. “In addition to accommodating a broad range of imaging studies, the new scanner will cut some MRI scan times by half, to 30 minutes. This benefits patients by reducing the time they spend in the scanner. It also means greater scheduling flexibility and increased access to equipment for our researchers.”
For more information: www.usa.siemens.com


 March 03, 2026
March 03, 2026 









