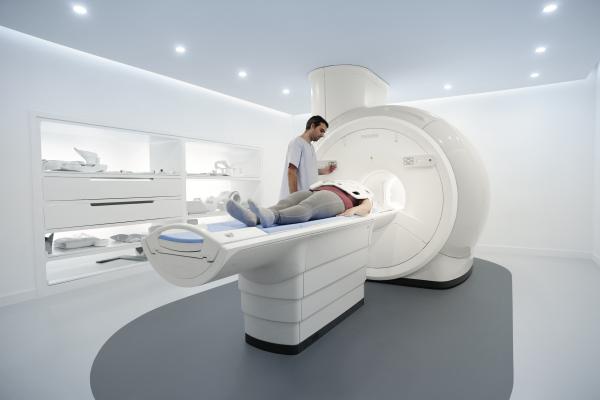
December 14, 2017 — Philips showcased the company’s latest magnetic resonance imaging (MRI) solutions at the Radiological Society of North America’s 2017 annual meeting, Nov. 26-Dec. 1 in Chicago. This includes their newest MR system, MR Prodiva 1.5T (which is still U.S. Food and Drug Administration [FDA] 510(k) pending) that provides enhanced clinical performance, workflow and patient experience. Philips also unveiled new solutions to drastically reduce exam times and elevate neuro-oncology.
Increased pressure on imaging procedures, complicated technology and longer wait times are hurdles that exist in many radiology departments globally. The new MR Prodiva 1.5T addresses this through Breeze Workflow, by providing a simplified, guided patient setup. Breeze Workflow consists of a flexible lightweight digital coil system to support fast patient setup. Combined with Philips’ dStream digital broadband technology, MR Prodiva 1.5T provides radiologists with consistent, high-quality images in support of improved patient care. It also helps manage costs through low transportation, installation and energy consumption expenses.
Patient discomfort during imaging exams can significantly impact outcomes, as evidenced by a recent study. MR Prodiva 1.5T features Philips’ Ambient Experience In-bore Connect, which elevates patient comfort by allowing them to personalize their environment with a visual theme, guiding them through the examination with instructions, and by reducing acoustic noise.
Philips also unveiled two new MR innovations that aim to accelerate MR procedures and improve neuro-oncology diagnoses:
- Reduce exam times – In healthcare today, reimbursements are declining and chronic conditions are leading to more MR procedures and longer wait times, increasing the pressure on the radiology department. However, accelerating scans with current technology can compromise image quality. To address this issue in MR, Philips has created Compressed SENSE (pending FDA 510(k)), an application that enhances productivity in imaging by increasing the data that is able to be pulled quickly from scans, including both 2-D and 3-D scans, all anatomical contrasts and all anatomies. It reduces imaging examination time and is applicable to the entire patient examination process.
- Elevate neuro-oncology – Philips’ new 3D APT (FDA 510(k) pending) helps address needs for new imaging contrast and biomarkers in neuro-oncology imaging. This contrast-free imaging solution uses Philips technology to effectively support neuro-oncology clinicians in providing a more confident diagnosis, and features a workflow that leverages optimized and standardized acquisition and visualization techniques.
For more information: www.usa.philips.com/healthcare


 March 03, 2026
March 03, 2026 









