April 6, 2016 — Researchers at University of California San Diego School of Medicine conducted a prospective study to assess the efficacy of two-dimensional magnetic resonance elastography (MRE) and a novel 3-D version in detecting advanced liver fibrosis from nonalcoholic fatty liver disease (NAFLD).
NAFLD comprises a group of liver disorders whose prevalence is widespread and rising. It’s estimated that at least one-third of Americans have NAFLD; among obese persons, the figure is 50 percent.
NAFLD, characterized by excessive accumulation of fat in liver cells, often begins without symptoms, but can progress to liver fibrosis (scarring), cirrhosis and cancer. The gold standard for detecting advanced fibrosis is a liver biopsy, but the procedure is invasive, results are subject to variable interpretation and possible adverse side effects include bleeding, pain and even death.
The study, published in The American Journal of Gastroenterology, assessed 100 patients (56 percent women) with biopsy-proven NAFLD. They found that both MRE technologies were highly accurate for diagnosing advanced fibrosis, with 3-D perhaps providing additional capabilities in some patients.
“3-D MRE is probably the most accurate non-invasive method to detect advanced fibrosis,” said Rohit Loomba, M.D., the study’s first author and director of the NAFLD Research Center at UC San Diego School of Medicine.
The researchers say the findings are encouraging because diagnosing NAFLD can be challenging. Current noninvasive techniques, such as molecular biomarkers in blood, are not sufficiently accurate for routine clinical use. Ultrasound-based methods have high failure rates, particularly in obese patients.
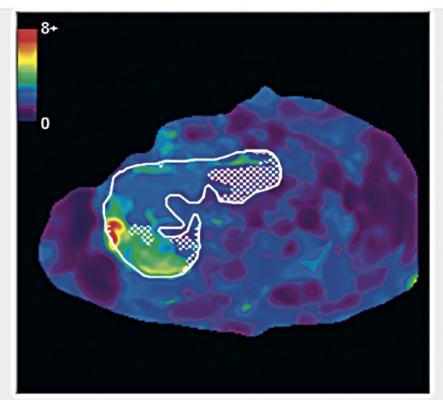
MRE is a specialized version of magnetic resonance imaging (MRI) that propagates mechanical shear waves in liver tissue. An algorithm creates images that quantitatively measure tissue stiffness — an indicator of fibrosis. The 2-D version of MRE is already commercially available and easily implemented on basic MRI systems in clinics. Three-dimensional MRE is more technically demanding and not yet widely available.
Loomba said the prospective study is the first to evaluate 3-D MRE for diagnosing advanced fibrosis in NAFLD patients. Both 2-D and 3-D were highly accurate, he said. The former was simpler to use, the latter offered improved assessment of spatial patterns and the ability to diagnose larger volumes of tissue. Unlike ultrasound, MRE accuracy did not appear to be impacted by obesity.
“These findings suggest that MRE could be used to enroll patients with advanced fibrosis into screening programs for cirrhosis as well as enrollment into clinical trials aimed at reversing fibrosis in the setting of advanced fibrosis,” he added.
Co-authors include Jeffrey Cui, Tanya Wolfson, William Haufe, Jonathan Hooker, Nikolaus Szeverenyi, Brandon Ang, Archana Bhatt, Kang Wang, Hamed Aryafar, Cindy Behling, Mark A. Valasek, Grace Y. Lin, Anthony Gamst, David A. Brenner, and Claude B. Sirlin, all at UC San Diego; and Meng Yin, Kevin J. Glaser, and Richard L. Ehman, Mayo Clinic.
Funding support for this research came, in part, from Atlantic Philanthropies Inc, the John A. Hartford Foundation, the Association of Specialty Professors, the American Gastroenterological Association and the National Institutes of Health.
For more information: www.nature.com/ajg


 February 13, 2026
February 13, 2026 









