September 12, 2016 — University of California Los Angeles (UCLA) doctors have found what may be an earlier and easier way to diagnose chronic traumatic encephalopathy (CTE). The disorder is thought to affect some former football players and others with a history of repetitive brain trauma.
Using a new software tool for analyzing magnetic resonance imaging (MRI) scans, the researchers detected the shrinkage of several key brain regions in a former football player with cognitive problems. The same pattern of brain changes is commonly seen in CTE cases that have been confirmed by autopsies after a person’s death.
While the findings from this single case report are preliminary, they raise the possibility that MRI scans could be used to diagnose CTE and related conditions in living people. At present, CTE can be diagnosed only by direct examination of the brain during an autopsy.
“Having an MRI-based technique for detecting this pattern of brain changes would help us a lot in assessing the brain health of athletes and others with histories of concussions,” said David Merrill, M.D., Ph.D., assistant clinical professor of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience and Human Behavior at UCLA.
Along with its potential benefits in diagnosing patients in the clinic, the MRI scanning technique could also help speed research of CTE, helping scientists better understand its prevalence in the population and in studying potential therapies, said Cyrus Raji, M.D., Ph.D., a senior resident in radiology at Ronald Reagan UCLA Medical Center.
Raji and Merrill are lead authors of the report, which appears online in the American Journal of Geriatric Psychiatry.
The case’s patient, with the pseudonym “Bob Smith,” is a former high school fullback who also saw action on defense and special teams, meaning he was on the field for much of entire games. During his three-year playing career Smith received hundreds of blows to the head, including at least one concussion in which he was knocked unconscious and, after regaining consciousness, jogged to the wrong sideline.
After finishing college, Smith married, raised a family and started a business with his wife. In his late 30s, he began to experience mood swings and difficulties with attention and impulse control. Eventually his problems affected his ability to work. He sought medical advice and was diagnosed with attention deficit hyperactivity disorder and bipolar disorder. Earlier this year, he sought medical help at Merrill’s UCLA Psychiatry Cognitive Health Clinic and Research Program. Standard neuropsychological testing confirmed Smith’s impairments in attention, impulse control and other measures of executive function, although it indicated that his memory was normal. An MRI scan showed no evidence of Alzheimer’s, stroke or dementia.
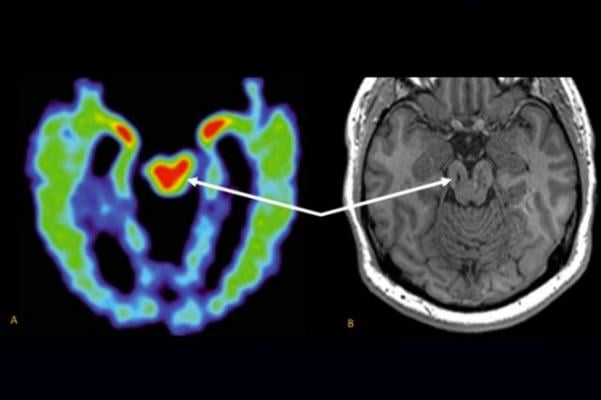
The MRI scan did reveal a few small lesions consistent with Smith’s history of brain trauma. Merrill and Raji also used a new U.S. Food and Drug Administration (FDA)-cleared MRI software analysis tool called Neuroreader to measure the volumes of 45 different regions of the patient’s brain.
“From one scan it can tell you which of those regions is abnormally large or small compared to a large database of normative brain volume,” Raji said of the software analysis tool. “Most importantly, we were able to see the progression of volume loss over time, because the patient had had another MRI done in 2012.”
The analysis revealed abnormally low volumes for Smith’s brainstem, his ventral diencephalon and frontal lobes. The shrinkage in these volumes was worse in the later scan, suggesting a progression of disease. On the whole Smith’s brain lost about 14 percent of its total gray-matter volume (the volume taken up by brain cells, not nerve fibers) during the four-year interval.
“The specific areas that were abnormally low in their volumes helped us understand that this patient’s cognitive symptoms were likely due to his history of traumatic brain injury,” Raji said.
Researchers are trying to develop other methods for diagnosing CTE in the living, including positron emission tomography (PET) scans based on radioactive tracers that bind to tau aggregates. But an MRI-based method, which uses no radioactive materials, would be safer and less expensive, Merrill said.
Merrill and colleagues now hope to do larger studies to analyze MRI data for people with histories of head trauma, which would help determine whether the technique is broadly useful in detecting CTE.
Other authors of the study included Jorge Barrio and Gary Small, M.D., of UCLA, and Bennet Omalu, M.D., of UC Davis.
For more information: www.ajgponline.org


 February 13, 2026
February 13, 2026 









