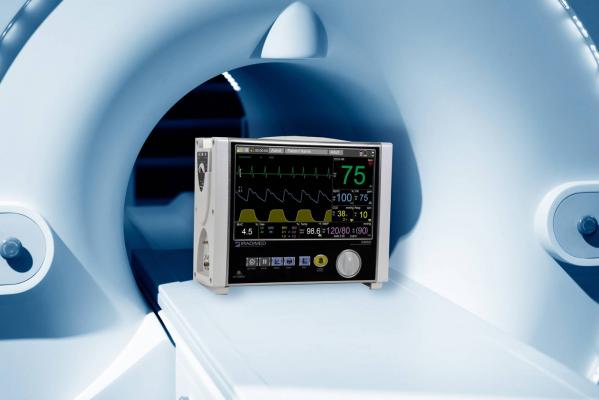
October 26, 2017 — Iradimed Corp. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its 3880 Magnetic Resonance Imaging (MRI)-compatible patient vital signs monitoring system.
The Iradimed 3880 introduces a compact and lightweight design to the MRI-compatible patient vital signs monitoring market, facilitating the transportation of patients from their critical care unit, to the MRI and back, resulting in increased patient safety through uninterrupted vital signs monitoring and decreasing the amount of time critically-ill patients are away from critical care units. It is the smallest, most portable MRI monitor on the market, according to the company.
The monitoring system has been designed with non-magnetic components and other special features in order to safely and accurately monitor a patient’s vital signs during various MRI procedures. The Iradimed 3880 monitor is rated for operations in magnetic fields up to 30,000 gauss, which means it can operate virtually anywhere in the MRI scanner room.
The features of the device include: wireless ECG with dynamic gradient filtering; wireless SpO2 using Masimo algorithms; respiratory CO2; non-invasive blood pressure; patient temperature, and; optional advanced multi-gas anesthetic agent unit featuring continuous Minimum Alveolar Concentration measurements.
For more information: www.iradimed.com


 January 28, 2026
January 28, 2026 









