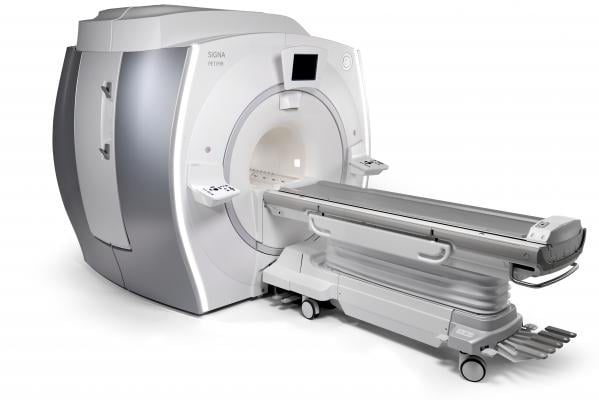
October 27, 2014 — At this year’s RSNA, GE Healthcare will showcase the Signa PET/MR, a time-of-flight (TOF) capable, integrated, simultaneous positron emission tomography/magnetic resonance (PET/MR) system.
The Signa PET/MR features GE’s new, exclusive MR-compatible silicon photomultiplier detector (SiPM) technology. This new digital detector is characterized by its enhanced sensitivity; it is up to three times more sensitive than GE Healthcare’s Discovery PET/CT 690. It also features fast coincidence timing resolution enabling TOF reconstruction. With TOF reconstruction, the arrival times of each coincident pair of photons are more precisely detected, and the time difference between them is used to localize the PET signal accurately. TOF helps to improve PET image quality with the potential for higher structural detail and signal-to-noise ratio. The Signa PET/MR is designed to be fully upgradable from a Discovery MR750w 3.0T.
New solutions with the DV25.0 Continuum Pak will be introduced for the Discovery line as well as Optima 450w and 450w GEM platforms at this year’s RSNA. The new features focus on the most challenging areas for today’s clinical users, including solutions to address productivity with up to 40 percent reduction of pre-scan time achieved through the new eXpress Prescan 2.0.
The next steps in patient comfort include a full Silent Suite that includes coverage across the body as well as diffusion imaging within 11dB(A). Features of the newly introduced Freedom Body Pak deliver computed tomography (CT)-like speed for body exams with the advantage of MR contrast. Further clinical enhancements include solutions for neurology as well as cardiac applications that help to address challenging patient situations through faster imaging and whole heart acquisitions in a single breath-hold.
The Signa PET/MR is 510(k) pending at the U.S. Food and Drug Administration (FDA) and not available for sale in the United States.
For more information: www.gehealthcare.com


 February 03, 2026
February 03, 2026 









