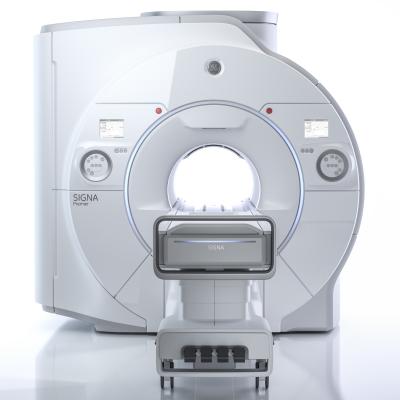
April 27, 2017 — GE Healthcare will showcase Signa Premier, its U.S. Food and Drug Administration (FDA) 510(k)-pending, wide bore 3.0T magnetic resonance imaging (MRI) system at the 25th International Society of Magnetic Resonance Imaging (ISMRM) Annual Meeting, April 22-27 in Honolulu, Hawaii.
Signa Premier is the result of a four-year collaboration with the National Football League (NFL) and research institutions around the world working to design new imaging tools. The new MRI system delivers a high performance with research-focused clinical capabilities designed particularly for neurological and oncology research and clinical work.
“The image quality we have seen to date from the Signa Premier is excellent and very consistent,” said Scott Reeder, M.D., Ph.D., vice chair of research and chief of MRI at University of Wisconsin-Madison (UW-Madison). UW-Madison, one of GE Healthcare’s top collaborators, is testing a research system of Signa Premier and has scanned approximately 100 subjects to date. “The high-fidelity gradients and RF systems of a scanner are key to obtaining high-quality, repeatable and reproducible quantitative measurements; this is increasingly important as we move from qualitative to quantitative imaging. Signa Premier combines the advantages of a wide bore system with an ultra-high-performance system and in some instances, is outperforming other ultra-high-performance systems with smaller bore sizes, particularly for diffusion-weighted applications. I believe the wide bore is going to improve access and comfort for patients at our hospital. We have also been able to improve the spatial resolution with very similar SNR performance as other ultra-high-performance systems,” said Reeder
This system is built on a platform with:
- A short-bore, high-homogeneity magnet;
- Powerful new gradients;
- A new digital RF transmit and receive architecture; and
- Cutting-edge applications, including cloud analytics.
Signa Premier is powered by SuperG gradient technology to improve diagnostic confidence for clinicians. The SuperG gradient coil introduces outstanding performance and superb stability, which is designed to deliver the performance of an ultra-high-performance, research-class 60 centimeter MRI system in a 70 centimeter bore MRI.
The RF technology of SIGNA Premier is designed to provide greater clinical performance and higher overall image quality, especially for data-intensive applications, based on GE’s Total Digital Imaging (TDI) architecture. A total of 146 independent channels allow the simultaneous combination of multiple ultra-high channel count surface coils for faster accelerations and uncompromised image clarity.
The TDI 48-channel Head Coil delivers clinical performance for virtually every patient. A fit- adaptable design addresses 99.99 percent of the population — including extremely large heads and short necks — while preserving high signal-to-noise ratio (SNR) and outstanding and sustainable image quality.
The new MRI system is based on the SignaWorks productivity platform, which is designed to improve productivity for radiologists and technologists. Signa Premier supports eight times faster productivity with HyperWorks, a new speed scanning tool. SignaWorks takes full advantage of the SuperG gradient technology and the TDI RF architecture to further advance and accelerate diagnostic outcomes, with signature applications such as MAGiC, HyperBand and HyperSense, while simultaneously improving patient throughput and ROI for clinicians. Further, the system runs on GE Healthcare’s Orchestra reconstruction platform that is now available through the company’s collaboration portal to allow researchers to compose their own reconstruction algorithms that can be easily integrated with the data acquisition on the scanner. Orchestra gives users the flexibility and potential to use the power of Signa Premier in new and innovative research.
For more information: www.gehealthcare.com


 March 09, 2026
March 09, 2026 









