December 15, 2022 — Fujifilm Healthcare Americas Corporation, a leading provider of diagnostic and enterprise imaging solutions, announced it will showcase its latest medical technology advancements and portfolio of medical imaging solutions at the 2022 Radiological Society of North America (RSNA) annual meeting, booth #1929, held November 27 - December 1 at McCormick Place in Chicago.
For the first time at RSNA, attendees will get to experience the following new medical innovations from Fujifilm:
-
Digital Radiography with Fujifilm’s new FDR D-EVO III G80i, the world’s lightest* long length detector with Fujifilm’s patented ISS and Hydro AG antibacterial coating. The detector is ideal for pediatric departments and hospitals that perform scoliosis, spine or leg procedures. The detector is now available for sale, shipments begin early Winter 2022. *Company investigation, compared to portable DR detectors of this size and type.
-
Advancements in Computed Tomography (CT) introducing SCENARIA View Focus Edition system – a new, premium scanner with an advanced Cardiac Motion Correction feature, called Cardio StillShot. Cardio StillShot feature helps clinicians capture clear images of the heart - even on the most challenging heart rhythms by using Advanced 4D Motion Estimation technology. The final images have dramatically improved effective temporal resolution down to as a little as 28 milliseconds, compared to 175 milliseconds without Cardio StillShot.
-
Women’s Health with Fujifilm’s ASPIRE Cristalle mammography system with Digital Breast Tomosynthesis (DBT). Patient experience enhancements such as its patented Comfort Paddles and new Comfort Comp feature are designed to make mammograms noticeably more comfortable. The recently introduced Comfort Comp feature reduces compression without notable change to compression breast thickness or glandular dose. Fujifilm also recently received 510(k) clearance for its contrast enhanced digital mammography (CEDM), an emerging modality that combines digital mammography with the administration of intravenous contrast material.
Fujifilm’s diagnostic imaging experts will also give booth visitors an update on additional new medical technologies that are pending regulatory approval in the U.S., including:
-
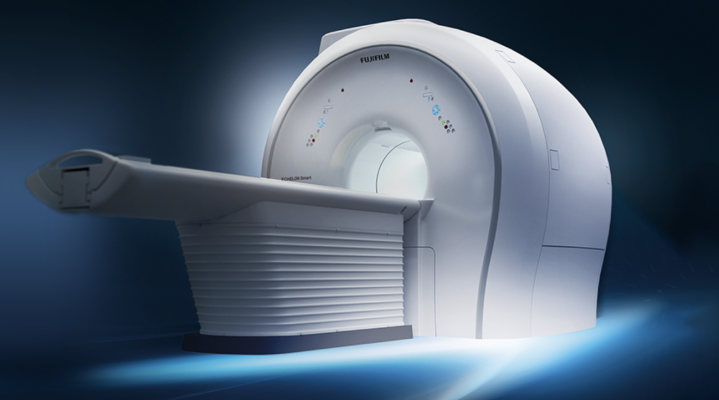
Next-generation MRI Introducing Fujifilm’s ECHELON Synergy System.* The powerful architecture, smart single-touch and on gantry controls, and AI driven reconstruction, make this system suitable for a wide range of anatomy and scans while helping speed procedure times and enhance patient experiences.
-
Battery-powered, ultra-portable digital X-ray Fujifilm’s FDR Xair.* This handheld lightweight x-ray solution has built-in lithium polymer battery, allowing users to shoot up to 100 images (utilizing Fujifilm specified conditions) in environments where there is no electricity and be used in remote out-of-hospital settings, like disaster response, patient and nursing homes, and more.
-
Artificial intelligence with BoneView*, an FDA cleared AI application manufactured by GLEAMER and distributed by FUJIFILM is designed to assist clinicians in the detection of orthopedic fractures. It uses advanced algorithms to detect and localize fractures on X-rays for adults 21 years or older – graphically highlighting areas of interest – as they are acquired and just before images transfer to radiologists for validation. Fujifilm’s X-ray systems in the U.S. will have the option to be equipped with Fujifilm’s new hardware kit called EX-Mobile enabling integration of the BoneView software onto workstations such as desktop and mobile acquisition consoles.
“When healthcare professionals are trying to diagnose medical conditions, imaging can provide a more accurate picture of what is going on inside the patient’s body. The detailed images from these scans show doctors where damage or abnormalities are, making it critical that they are using the best equipment,” said Henry Izawa, president and chief executive officer of FUJIFILM Healthcare Americas Corporation. “We’re looking forward to meeting with RSNA attendees to demonstrate how our cutting-edge solutions offer a powerful combination of high image quality and easy-to-use features, empowering clinicians to feel confident in their diagnosis and treatment plans for their patients.”
*Technologies with an asterisk will be available in the U.S. upon completion of regulatory requirements.
For more information: https://holdings.fujifilm.com/


 March 06, 2026
March 06, 2026 









