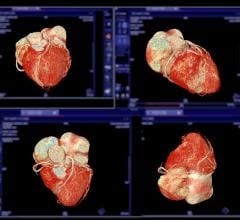
March 28, 2008 - Toshiba America Medical Systems Inc. received FDA clearance for two new CT clinical applications, SURECardio Prospective and Variable Helical Pitch (vHP), designed to improve throughput and enhance workflow, while further reducing contrast and radiation dosage.
The applications are available for new Toshiba Aquilion 32- and 64-slice CT systems.
Toshiba's SURECardio Prospective aims to help reduce patient dose by up to 80 percent during coronary CTA exams by using a helical acquisition technique to provide one continuous image instead of multiple images produced by the current step-and-shoot techniques. SURECardio Prospective is said to automatically detect and adjust to patients with arrthymia, for quicker, more conclusive exam results.
It is also engineered to automate scan parameters and reconstruction based on the patient's heart rate. It also speeds exam time and eliminates the need to use additional contrast as with the step-and-shoot method.
Variable Helical Pitch (vHP) increases workflow and efficiency by enabling physicians to complete an exam of more than one anatomical region consecutively - without stopping to alter the helical pitch of the exam. With vHP, according to the manufacturer, the patient's gated heart and non-gated body can be imaged during a single exam, reducing patient radiation and contrast dose and saving critical time for the imaging center or hospital. This feature targets the needs of trauma centers, in particular.
vHP also reportedly saves time by producing one comprehensive image instead of two separate images that would result from separate scans. This eliminates the task of piecing together separate images.
Both clinical applications are available to purchase independently and will be offered with all new Aquilion 32 and 64 CT systems.
For more information: www.medical.toshiba.com


 February 06, 2026
February 06, 2026