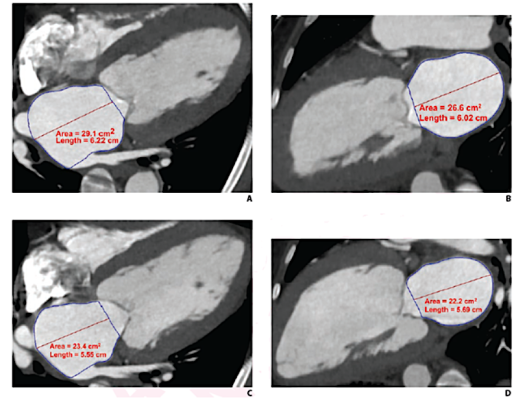
Cardiac CTA derived left atrium emptying fraction (LAEF) improves predictive performance of established clinical risk scores and may be used to assess TAVR patient risk during pre-procedure workup and post-procedural surveillance. These images show LAVmax (A and B), LAVmin (C and D), and LAEF using cardiac CTA. LAEF was calculated as (LAVmax-LAVmin)/LAVmax x 100. Given LAVmax of 109 mL and LAVmin of 80 mL, the LAEF was calculated to be 27%. Image courtesy of AJR.
October 14, 2021 — Cardiac computed tomography angiography (CTA) derived left atrium emptying fraction (LAEF) improves predictive performance of established clinical risk scores and may be used to assess patients’ risk during pre-transcatheter aortic valve replacement (TAVR) workup and post-procedural surveillance. This is according to a new study published in the American Roentgen Ray Society (ARRS) American Journal of Roentgenology (AJR).[1]
“LAEF derived from preprocedural cardiac CTA independently predicts mortality in patients with severe aortic stenosis undergoing TAVR,” concluded corresponding author Joseph Schoepf, M.D., from the Medical University of South Carolina’s Heart and Vascular Center.
Aortic stenosis (AS) is an obstructive valvular disorder associated with changes in the left ventricle (LV) and left atrium (LA). Chronically elevated LV pressures lead to LV myocardial hypertrophy and fibrosis, which consequently lead to LA enlargement and remodeling to maintain adequate filling of the LV. These changes in the LA function can be used as an early indicator of atrial remodeling and can be demonstrated by reduced LAEF, and increased maximum (LAVmax) and minimum (LAVmin) LA volume. The authors of the study said parameters of LA function are most commonly assessed using transthoracic echocardiography (TTE), but can also be evaluated with cardiac MRI and cardiac CCTA. In patients with severe AS undergoing TAVR, LA dilatation assessed by TTE is associated with poor outcome, which raised the question if CTA could be used to assess TAVR patients.
CT is already required for preprocedural workup of patients undergoing TAVR and provides not only information on the arterial access route, but also anatomic and morphologic assessment of the aortic valve for annular prosthesis sizing, positioning of the valve and determination of risk of annular injury or coronary occlusion. Information on preprocedural CTA can also provide information on volumes and function of the LA with appropriate post-processing and this study looked at whether the data could be leveraged to predict outcomes in patients undergoing TAVR.
Schoepf and colleagues’ retrospective single-center study included 175 patients with severe aortic stenosis (92 male, 83 female; median age, 79 years) who underwent cardiac CTA for clinical pre-TAVR assessment. Maximum and minimum left atrium volumes were calculated using biplane area-length measurements, and the values were indexed to body surface area: LAVImax and LAVImin, respectively.
In their sample, a reduced LAEF independently predicted all-cause mortality within 24 months post-procedure (hazard ratio 0.97 [0.94–0.99]; p=.02). Moreover, when incorporating LAEF, the c-index of the Society of Thoracic Surgeons Predicted Risk of Mortality significantly increased from 0.64 to 0.70.
Acknowledging that atrial parameters are more commonly assessed using transthoracic echocardiography, both atrial volume and atrial function can be reliably assessed using cardiac CTA, “which now represents the gold standard for preprocedural planning in patients undergoing TAVR,” the authors of this AJR article added.
For more information: arrs.org
Reference:


 March 09, 2026
March 09, 2026