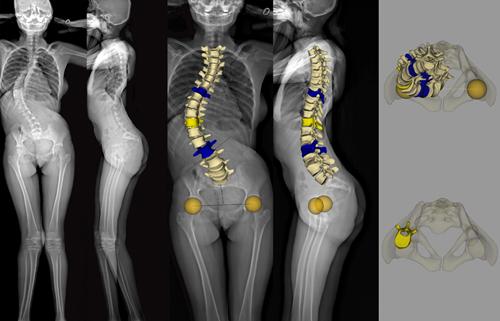
EOS imaging, a in 2D/3D orthopedic medical imaging, announced today that the U.S. Food and Drug Administration (FDA) has approved spineEOS, an online 3D planning software for spine surgery based on EOS stereo-radiographic 2D/3D imaging. The FDA approval of spineEOS allows EOS to expand its presence in the large and growing U.S. spine market, in particular the complex spine and thoraco-lumbar fusion surgery markets, which include approximately 400,000 patients per year and represent more than $3 billion in orthopedic implant value[1].
The spineEOS online 3D planning software is intended for adults suffering from degenerative or deformative spine conditions, as well as for pediatric patients with Adolescent Idiopathic Scoliosis. It allows a surgeon to create a treatment plan to achieve optimal sagittal alignment from pelvic and vertebral 3D data obtained in the functional standing position from an EOS exam. The planned surgery and virtual post‑correction 3D anatomy can be used to precisely plan for the 3D shape and length of the spinal implants. It can also be shared pre-operatively to engage the patient in the intended course of therapy, and is accessible in the operating room through a custom planning report.
Marie Meynadier, CEO of EOS imaging, said, “We believe that patients’ spines are complex 3D systems that need a personalized 3D planning of the intended surgery. We’re excited about the interest we’ve seen in our spineEOS planning software from surgeons inside and outside of our current installed base, as well as from our industry partners. We look forward to this next important step toward our broader goal of expanding our EOSapps planning software suite to better connect imaging to patient care.”
For more information: www.eos-imaging.com


 February 09, 2026
February 09, 2026 









