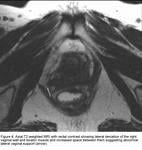
Axial T2 weighted MRI showing lateral deviation of the right vaginal wall.
November 24, 2009 - Dynamic MRI assists in diagnosing pelvic organ prolapse, which is a condition often undiagnosed on static MRI and physical examinations.
In the study, which appears in the December issue of the American Journal of Roentgenology, researchers conducted the dynamic MRI study, while the patient performed a straining maneuver, such as bearing down. Static MRI was performed while the patient is at rest.
The study, performed at NYU Langone Medical Center in New York, included 84 women with lower urinary tract symptoms who underwent dynamic and static MRI scans for a suspected urethra abnormality. Ten of the 84 patients were found to have an abnormality of the urethra. Thirty-three of the patients were diagnosed with pelvic organ prolapse, of whom 29 were diagnosed exclusively on dynamic imaging, explained lead author of the study, Genevieve L. Bennett, M.D., assistant professor of radiology at NYU Langone Medical Center.
"Dynamic imaging allows for the detection of pelvic organ prolapse, which may not be evident at rest but only detected when the woman strains," said Bennett.
Researchers concluded that in women with lower urinary tract symptoms who undergo MRI for evaluation of a suspected urethra abnormality, the addition of dynamic MRI permits detection of pelvic organ prolapse that may not be evident on static at rest images and that may also go undetected at physical examination.
For more information: www.ajronline.org/


 February 13, 2026
February 13, 2026 









