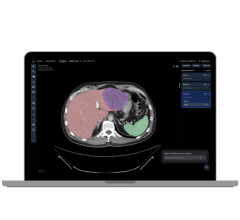
October 5, 2018 — Body Vision Medical, a medical device company specializing in lung cancer diagnostics, announced the launch of its LungVision platform. The platform will be presented at the CHEST conference, Oct. 7-10 in San Antonio, Texas.
"Body Vision provides a great tool that has changed the way we approached peripheral nodules. Its unique technology creates augmented reality with the ability to see fluoroscopically invisible lesions and the pathways under the live fluoro, while tracking a patient breathing," said D. Kyle Hogarth, M.D., FCCP, president of the Society for Advanced Bronchoscopy, associate professor of medicine, director of bronchoscopy at the University of Chicago. "Once the technology has guided us to the lesion, we confirm the lesion's relationship to the airway with radial-EBUS [endobronchial ultrasound]. We then use our off-the-shelf biopsy instruments via the LungVision catheter. The augmented fluoro image, integrated with the radial EBUS images, allows us to obtain tissue samples with continuous real-time confirmation of location. The beauty of it all is how simple and elegant the technology is."
Body Vision CEO Dorian Averbuch said the product targets small pulmonary nodules that, if diagnosed early, can be resected to cure lung cancer. The technology provides step-by-step guidance through planning, navigation and lesion localization during biopsy.
The clinical experience with the LungVision platform will be featured through the following panel presentations during the CHEST conference at San Antonio, TX:
- Sun. Oct. 7
- Krish Bhadra, M.D., Novel Augmented Fluoroscopic Imaging Platform Accuracy Assessment during Navigation Bronchoscopy in Simultaneous Comparison with Cone Beam CT and Radial EBUS
- D. Kyle Hogarth, M.D., FCCP, A Novel Endobrachial Fluoroscopic Navigation and Localization System: a summary of a multi-center LungVision Trial
- Tue., Oct. 9
- Patrick E. Whitten, M.D., FCCP, Augmented Endobronchial Fluoroscopic Navigation and Localization System: Integration of Multimodalities and Biopsy Tools to Increase Diagnostic Yield
For more information: www.bodyvisionmedical.com


 February 04, 2026
February 04, 2026