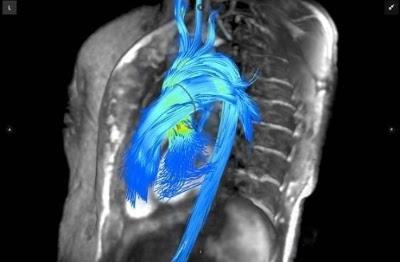
November 21, 2016 — Arterys will showcase its recently U.S. Food and Drug Administration (FDA) 510(k)-cleared Arterys Software platform at the upcoming Radiological Society of North America (RSNA) 2016 meeting, Nov. 27-Dec. 1 in Chicago. The company will also present new data on cardiac clinical applications using the Arterys 4-D Flow software during the RSNA poster sessions.
Arterys received 510(k) clearance from the FDA for its Arterys Software earlier this month, paving the way for use in clinical settings for the quantification of cardiac flow. This includes 4-D Flow and 2-D Phase Contrast workflows, and cardiac function measurements. Arterys plans on launching the product in the United States through a partnership with GE Healthcare's ViosWorks product.
Arterys representatives will be on hand at the GE Healthcare booth to help demonstrate how the product integrates with ViosWorks to provide comprehensive visualization and quantification of the cardiac anatomy, function and flow. Powered by the Arterys software, ViosWorks will be the first clinically available cardiovascular solution that delivers cloud-based, real-time processing of images with resolutions previously unattainable, according to the company.
Arterys will also host three expert sessions in the Quantitative Imaging Reading Room to provide 4-D Flow magnetic resonance imaging (MRI) software demonstrations on applications of cardiac flow 4-D MRI, and cardiac volumetry and function features. Arterys medical experts will also discuss how deep learning can be applied to automate measurements and provide accurate and consistent cardiac flow and function.
For more information: www.arterys.com


 February 02, 2026
February 02, 2026 









