Medivis announced that its augmented reality (AR) technology platform for surgical applications, SurgicalAR, has received 510(k) clearance for clinical use in the operating room by the U.S. Food and Drug Administration (FDA). The New York City-based medical technology company will commence the…
Procedure Navigation Systems
News and new technology innovations for procedure navigation systems can be found on this channel.
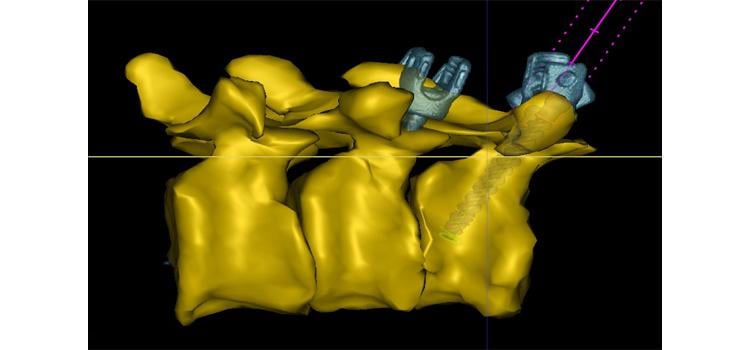
January 18, 2022 – Philips Healthcare announced physicians will now have access to advanced new 3D image guidance ...
October 24, 2019 — EOS imaging announced the first patient cases performed with its hipEOS 3.0 surgical planning ...
October 17, 2019 – International medical imaging information technology (IT) and cybersecurity company Sectra is ...
September 26, 2019 — Digital medical technology company Brainlab unveiled Loop-X, which it calls the first mobile ...
September 25, 2019 — A UCLA-led study has found that using three-dimensional virtual reality (VR) models to prepare for ...
June 10, 2019 — Medivis announced that its augmented reality (AR) technology platform for surgical applications ...
April 26, 2019 — Pickup Family Neurosciences Institute at Hoag in Newport Beach, Calif., announced the addition of the ...
February 14, 2019 — Medical imaging and visualization company Medivis officially unveiled SurgicalAR, its augmented ...
February 5, 2019 — Medtronic is recalling the Synergy Cranial Software and StealthStation S7 Cranial Software used with ...
November 20, 2018 — Stryker's Advanced Guidance Technologies business (formerly known as Stryker's Navigation business) ...
June 13, 2018 – EOS imaging announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) ...
January 22, 2018 — On Jan. 8, 2018, Robotics Outpatient Center, Los Angeles (ROC-LA), became the first outpatient ...
October 26, 2017 — The Netherlands Cancer Institute (NKI) announced it has received funding from the Dutch Cancer ...
October 20, 2017 — Surgeons at The Johns Hopkins Hospital have for the first time used a real-time, image-guided robot ...
October 9, 2017 — A clinical trial conducted at Stanford University Medical Center published online in Anesthesia & ...
September 13, 2017 — ClaroNav announced that it has received U.S. Food and Drug Administration (FDA) 510(K) clearance to ...
September 6, 2017 — The U.S. Food and Drug Administration (FDA) has cleared TrueFusion, a new cardiovascular application ...
July 6, 2017 — The U.S. Food and Drug Administration (FDA) recently issued a safety communication alerting healthcare ...
April 6, 2017 — Image Diagnostics Inc. (IDI) recently introduced the ilex55 as the world’s first large field 4k mobile ...
March 21, 2017 — EchoPixel recently announced progress in the clinical adoption of its True 3D virtual reality software ...


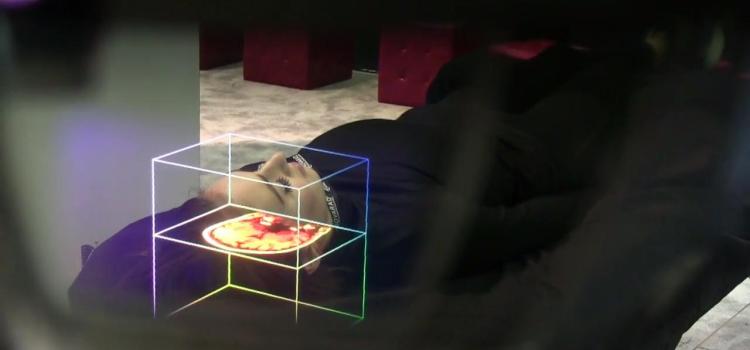


 January 18, 2022
January 18, 2022