November 1, 2013 —
Clinical trial results demonstrated that a noninvasive
coronary computed tomography angiography (CTA)-based test accurately assesses coronary artery disease (CAD) with results closely matching those of invasively measured fractional flow reserve (FFR), and may inform potential revascularization treatment options, including angioplasty and coronary artery bypass surgery (CABG), better than current methods. The findings of the HeartFlowNXT trial were presented at the 25th annual
Transcatheter Cardiovascular Therapeutics scientific symposium (TCT 2013).
Current guidelines for the management of stable CAD recommend non-invasive ischemia testing such as treadmill testing, single photon emission computed tomography (SPECT), stress echocardiography or cardiac magnetic resonance before invasive coronary angiography (ICA) or coronary revascularization is considered. An invasive test, FFR, is considered the gold standard for lesion-specific coronary revascularization decisions in patients with stable CAD.
However, the current noninvasive testing options such as treadmill testing, SPECT and stress echocardiography correlate poorly to FFR, and thus selection of patients for invasive angiography and coronary revascularization is often inaccurate.
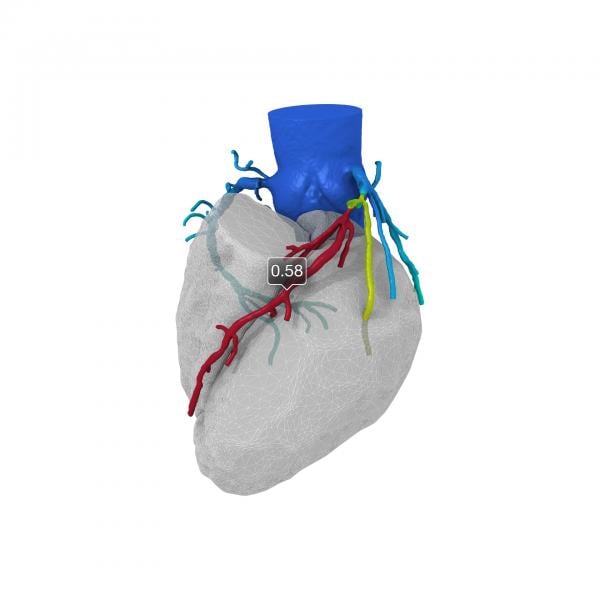
The HeartFlowNXT trial was a blinded, prospective core lab adjudicated trial that investigated FFR-CT, a new, noninvasive FFR calculation derived from CTA images and using high-performance computing to create a 3-D map of the coronary arteries showing locations of obstructions and FFR-CT values.
The primary study objective was to compare per-patient diagnostic performance of FFR-CT compared to coronary CTA alone for the diagnosis of at least one hemodynamically significant stenosis using direct measurement of FFR (? 0.8) as the reference standard. The trial also measured per-vessel and per-patient diagnostic accuracy, sensitivity and specificity as well as positive and negative predictive values of FFR-CT.
The prospective study enrolled 254 patients scheduled to undergo non-emergent clinically indicated invasive angiography due to suspected CAD.
The FFR-CT data matched closely with invasively measured FFR. The area under the receiver operating characteristic curve (AUC) for FFR-CT (? 0.8) was 0.82 versus 0.63 for coronary CTA (p < 0.0001) with invasive FFR as the reference standard. Per-patient sensitivity and specificity were 86 percent and 79 percent for FFR-CT versus 94 percent and 34 percent for coronary CTA and 91 percent and 51 percent for invasive coronary angiography (lumen reduction > 50 percent).
“Fractional flow reserve non-invasively calculated from coronary CTA data sets matches closely with invasively measured FFR and allows for identification of patients with hemodynamically significant coronary lesions with good accuracy. When compared to both coronary CTA and ICA, FFR-CT led to a significant reduction in the proportion of false-positive results,” said Bjarne Nørgaard, M.D., Ph.D., Aarhaus University Hospital, Denmark and lead investigator of the study. “The addition of FFR-CT to coronary CTA allows for a comprehensive anatomic and functional assessment of coronary artery disease.”
Limits of the Current Technology
CT-FFR may one day eliminate the need to refer patients for diagnostic catheter angiographies. However, today, users of the HeartFlow technology say it requires sending the CT DICOM dataset to the company in California. It then takes the supercomputing power of the HeartFlow computer system about a day to process the data and render a report. While this may eliminate CT-FFR's use for acute chest pain patients, it offers an option for patients considering elective PCI. Coronary CT experts say computing speed and power doubles every couple years, which will eventually enable CT-FFR to be processed onsite in minutes, instead of days or hours.



 February 09, 2026
February 09, 2026 









