Getty Images
The term nuclear medicine is associated with the diagnostic procedures single-photon emission computed tomography (SPECT), positron emission tomography (PET) and other hybrid imaging modalities such as SPECT/computed tomography (CT) or PET/CT. However, the therapeutic radiopharmaceutical field is making a rapid progression in addressing a largely unmet demand in finding a cure for very difficult to treat diseases, such as cancers and infectious diseases. Currently, the radiotherapy market is estimated to be about one third the size of the nuclear medicine/radiopharmaceuticals market. However, this market segment is expected to grow in a double-digit compound annual growth rate (CAGR) in coming years due to an increase in the application of therapeutic radionuclides for various conditions. Radiotherapy mainly consists of beta radiation therapy, alpha radiation therapy and brachytherapy.
Theranostics
The current beta radiation therapy is dominated by lutetium (Lu-177), yttrium (Y-90) and iodine (I-131) isotopes which together generated an estimated $1 billion in revenue during 2020, and the opportunity is expected to grow exponentially to reach $4 billion in the coming 6 to 7 years. The rapid adoption of theranostics, a combination of diagnostic and therapeutic isotopes where one radioactive isotope is used in the diagnosis and the second radioactive isotope delivers therapy to treat the disease through the use of small molecule or antibody that preferably target cancer cells, is propelling this growth. The current theranostic market is dominated by gallium (Ga-68) and beta emitter lutetium (Lu-177) (Lutathera) with an estimated $492.8 million in revenue during 2020. The prospects of reaching blockbuster status in the near future has increased interest in theranostic development among major pharmaceutical companies.
An analysis by IQ4I Research indicated that close to $550 million was invested by various governments, venture capitals and pharmaceutical companies during 2018-2020 in the development of advanced therapies and for increasing market access of various isotopes.
Several universities and companies are working on the development of therapies for patients suffering from an advanced stage of cancers. For example, Telix Pharmaceuticals Limited (Australia) is working on a theranostic pair consisting of gallium (Ga-68), zirconium (Zr-89), Iodine (I-124) for imaging and lutetium (Lu-177) for targeted therapy of prostate cancer, renal cancer and iodine (I-131) for glioblastoma. Similarly, Novartis (AAA) is working on gallium (Ga-68) based radioligand imaging molecules and lutetium (Lu-177) therapy molecules aimed at the treatment of prostate cancer. Research and development are also undergoing as an investigational treatment of neuroblastoma, small-cell lung cancer and somatostatin-receptor-positive small bowel carcinoid tumors. An analysis of clinical trials that are currently underway indicates that among beta emitters, about 51 clinical trials are centered on lutetium (Lu-177).
Yttrium
Yttrium (Y-90) is the most common beta emitter used in different forms for the treatment of cancers affecting humans. It is used as labeled antibodies for the treatment of non-Hodgkin’s lymphoma (Zevalin) manufactured by Spectrum Pharmaceuticals, Inc. Similarly, it is used in the radioembolization process for treating unresectable hepatocellular carcinoma (HCC) in the form of microspheres, which are manufactured and marketed as TheraSphere (glass-based) (Boston Scientific) and SIR-Spheres (resin based) (Sirex Medical). In June 2020, Sirtex Medical reached the 100,000th patient dose of SIR-Spheres Y-90 resin microspheres, a treatment for patients with liver cancer, Hepatocellular Carcinoma (HCC). More than 1,000 healthcare providers and hospital systems across the globe offer this treatment.
Strontium
Strontium (Sr-89) is one more high growth potential beta emitter. Currently it is prescribed as an intravenous injection of Sr-89 chloride solution which accumulates in the sites where the osteoblastic activity is high in the bone. Sr-89 acts as calcium analogs and is readily absorbed by bone minerals, and helps in the reduction of pain in bone metastases especially in prostate cancer patients. Strontium-89 in combination with zoledronic acid acts as an anti-cancer therapy and was effective for breast cancer patients with painful bone metastases. In February 2020, Q Biomed Inc. launched its U.S. FDA-cleared strontium-89, a non-opioid drug used to treat the pain associated with cancer that has metastasized to bone.
The alpha-emitting radioisotopes have the distinct advantage of lowering the risk of collateral radiation crossfire because of their low cell penetration diameter of 50-90 µm. The linear energy transfer (LET) of alpha particles, approximately 25-230 keV/µm, is about 100 to 1,000 times greater than the average LET of beta particles. This higher LET results in a greater potential for biologic damage by alpha particles as compared to beta particles, and other lower LET radiations. Combined, the small range and high LET of alpha particles allow for relatively focal radiation therapy with high potency, with the radionuclide quite precisely to the region of interest. Currently, Xofigo, radium (Ra-223) chloride, marketed by Bayer Healthcare Pharmaceuticals, is the only approved alpha particle emitter by the FDA and the European Medicines Agency (EMA) for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases and no known visceral metastatic disease. Revenue generated by Xofigo in 2020 was close to $300 million.
Actinium
Actinium (Ac-225) is of particular interest as it has the distinct advantage of having a long 10-day half-life that allows for centralized production and distribution solving logistical and quality control issues, when compared to isotopes with shorter half-life that needs to be produced at radiopharmacy. Actinium is used for the production of its daughter product bismuth (Bi-213) in a reusable generator as an agent for radiation therapy. Its daughter isotopes emit alpha and beta rays, but not high-energy gamma rays. Alpha-immunotherapy using Ac-225 and its daughter isotope are well-matched for targeting micrometastatic disease (cancers that spread) and blood-borne cancers (leukemia) because of their unparalleled cytotoxicity toward targeted cells and lack of toxicity for untargeted normal tissue. Targeted alpha therapy (TAT) has an advantage over beta therapy in that it does not rely on the generation of free-radicals for the generation of DNA damage, as in the case of beta emission therapies, so a major mechanism of resistance to radiation treatment can be bypassed.
Currently, there are three sources of thorium (Th-229) worldwide that allows the production of clinically relevant activities of actinium (Ac-225) or Bi-213. This includes the Directorate for Nuclear Safety and Security of the JRC of the European Commission in Karlsruhe, Germany (formerly known as Institute for Transuranium Elements), Oak Ridge National Laboratory (ORNL), USA, and at the Institute of Physics and Power Engineering (IPPE) in Obninsk, Russia. It has been reported that from 2019 onward a very significant increase in the availability of Th-229 will be generated through the extraction of it from legacy wastes stored within the U.S. Department of Energy.
Brachytherapy
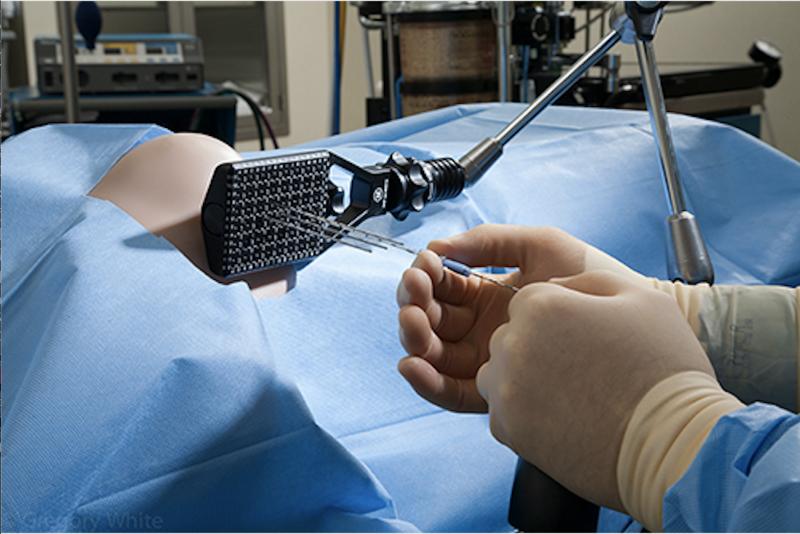
In brachytherapy, radioactive “seeds” are carefully placed inside the cancerous tissue, which is about the size of a grain of rice, and the radiation travels only a few millimeters to kill nearby cancer cells. Seed implantation brachytherapy can either be permanent or temporary, and is generally a very safe procedure. Cancers treated with temporary implants include many gynecology cancers, and cancer treated with a permanent implant is mostly prostate cancer. The radioactivity of the seeds decays with time, and the isotopes used in brachytherapy are iodine (I-125), cesium (Cs-131), iridium (Ir-192), palladium (Pd-103), cobalt (Co-57) and ruthenium (Ru-106).
The choice of seed to be implanted depends on parameters such as type of radiation emitted and the duration. For instance, palladium seeds (Pd-103) produce radiation more rapidly and over a shorter period, which makes it best suited to treat faster growing and more aggressive tumors of prostate cancer. I-125 seeds, which decay gradually, are recommended for the treatment of slow-growing tumors. Cs-131 are used to treat gynecological cancers, brain tumors, sarcomas and other aggressive and recurrent tumors and prostate cancer.
The emergence of therapeutic radiopharmaceuticals and its adoption in the cancer practice provide one more weapon in combating cancer, which has emerged as a major cause of death worldwide.
Vinay Shivaprasad is a post-graduate with experience in market research in various capacities. He has worked on more than 50 projects in various domains covering pharmaceutical,
biotechnology, medical devices and healthcare IT.


 February 11, 2026
February 11, 2026 









