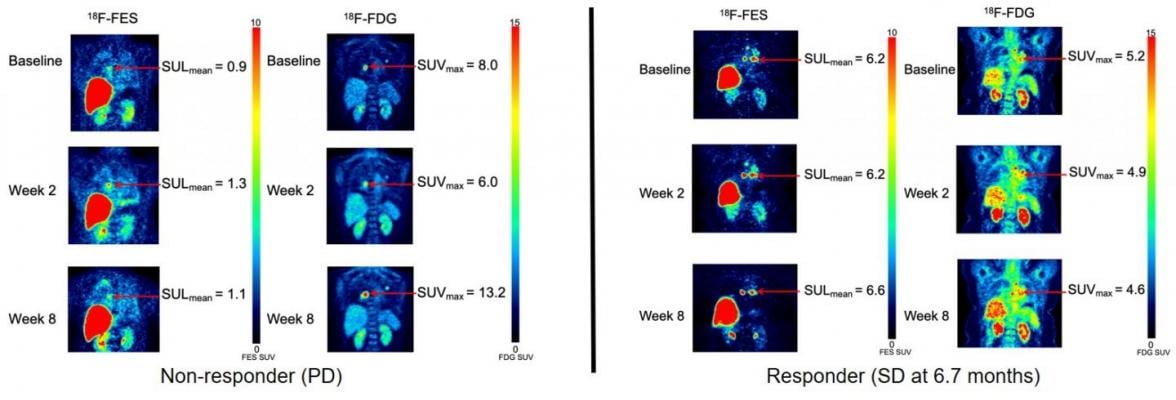
F-18 FES PET images of patients with ER+/PR+/HER2- invasive ductal carcinoma. Left panel: Progressive disease seen at the 8-week time-point in a patient on sequential therapy. Right panel: Stable disease through all 3 time-points, remaining on study therapy for 6.7 months until disease progression on combined vorinostat aromatase inhibitor therapy. Image created by Lanell M Peterson, Research Scientist, University of Washington Medical Oncology, Seattle WA.
February 22, 2021 — Molecular imaging can successfully predict response to a novel treatment for ER-positive, HER2-negative metastatic breast cancer patients who are resistant to hormonal therapy. According to research published in the February issue of the Journal of Nuclear Medicine, positron emission tomography (PET) imaging using an imaging agent called 18F-fluoroestradiol can help to determine which patients could benefit from treatments that could spare them from unnecessary chemotherapy.
Nearly two-thirds of invasive breast cancers are ER-positive, and endocrine therapy is the mainstay of treatment for these tumors because of its favorable toxicity profile and efficacy. Should cancer progress in these patients, however, salvage endocrine therapy with molecularly targeted agents or chemotherapy can help.
"In some ER-positive breast cancer patients, cancer progression can be a result of a gradual resistance to endocrine therapy," noted Hannah M Linden, MD, FACP, Athena Distinguished Professor and breast medical oncologist at the University of Washington Fred Hutchison Cancer Research Center and Seattle Cancer Care Alliance in Seattle, Washington. "Histone daecetylase inhibitors (HDACIs) have been proposed as a mechanism to reverse endocrine resistance, and clinical studies have shown promising results when combining endocrine therapy with HDACIs to restore endocrine sensitivity."
To further explore the efficacy of this combination therapy, researchers designed a study in which 18F-FDG PET imaging with 18F-fluoroestradiol was conducted on patients receiving vorinostat, a potent HDACI, along with an aromatase inhibitor, a type of endocrine therapy. Scans were performed at baseline, week two and week eight of the study. Subjects included ER-positive/HER2-negative breast cancer patients who had previously responded well to endocrine therapy while on an aromatase inhibitor. Eight of the study participants were treated with vorinostat followed by an aromatase inhibitor, while 15 were treated with both at the same time.
After eight weeks, eight patients had stable disease, and six of the eight patients were stable for more than six months. Higher baseline 18F-fluoroestradiol uptake was associated with longer progression-free survival. 18F-fluoroestradiol uptake did not systematically increase with vorinostat exposure, indicating no change in regional ER estradiol binding, and 18F-FDG uptake did not show a significant decrease, which is expected with tumor regression.
"We test ER expression in a metastatic biopsy once at the beginning of the patient's journey," explained Linden, "and we make decisions all along--when to give chemotherapy, when to use endocrine therapy, whether or not to use targeted agents--based on that one measurement. Since we know that ER expression can fluctuate, imaging with 18F-fluoroestradiol at various time points could help clinicians predict response to endocrine therapy and select optimal treatment in the future."
For more information: www.snmmi.org


 March 06, 2026
March 06, 2026 









