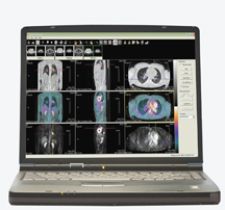
Cedara's I-Response features fDM, I-Response lesion and PET analysis tools for evaluation.
The ability to evaluate the efficacy of a given treatment for cancer is critical in achieving the best prognosis and care for patients. However, most standard methods of treatment response evaluation rely on visualizing a change in tumor size, which often occurs months after treatment begins. Determining the effectiveness of treatment earlier could enable physicians to adapt treatment, which could likely change prognosis and outcome. Research underway at the University of Michigan on functional Diffusion Mapping (fDM) may prove extremely effective in assessing treatment response early on.
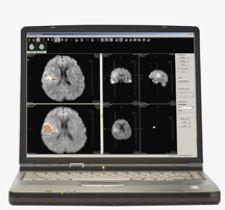
Daniel A. Hamstra, M.D., Ph.D., clinical lecturer in radiation oncology and in the center for molecular imaging at the University of Michigan at Ann Arbor has worked with Al Rehemtulla, Ph.D., and Brian Ross, Ph.D., since 1994 in developing a new imaging technique called fDM used with magnetic resonance imaging (MR) to evaluate tumor response to treatment in the early stages. The concept behind fDM is that tumor cells slow the diffusion of water, and as more cells die, the speed of water diffusion increases. Specifically, this technique uses diffusion weighted MR images to look at the microscopic, cellular motion of water and then diffusion mapping compares those images spatially rather than taking an absolute value. By taking these measurements, which relate the death of tumor cells in response to treatment, physicians may be able to evaluate treatment in a matter of weeks as compared to months. Being able to assess changes earlier, it is hoped that treatment regimens will become more personalized and become better tailored to particular patient needs, thereby leading to improved patient care.
In 2004, the researchers at the University of Michigan investigated whether fDM could be used as a biomarker for early prediction of treatment response in brain cancer patients. “Essentially we and many other groups [of researchers] use diffusion MR to measure the mobility of water within tissues. Our group has been looking at changes in diffusion MR, which would correlate with response treatment. The functional diffusion map is one way to quantify those changes. Prior to this, people have looked at average values over the tumor. fDM looks at specific tumor areas and that’s the essential difference,” according to Dr. Hamstra.
In their research with brain tumor response, images were taken with both standard and diffusion MR before treatment and three weeks after treatment. These images were calculated and displayed as a “functional diffusion map,” which was able to predict patient response at three weeks from the start of treatment, demonstrating that early changes in tumor diffusion values could be used as a prognostic indicator of subsequent volumetric tumor response. Overall, fDM analysis provided an early biomarker for predicting treatment response in brain tumor patients.
Despite the groundbreaking discovery of fDM in brain tumor assessment, clinical data involving humans does not exist that demonstrates the effectiveness of fDM in assessing other types of cancer response to treatment, according to Dr. Hamstra. Until recently, research at the University of Michigan was limited to assessing brain cancer response. Now, their research has expanded to include primary breast cancer, primary head and neck cancer and metastatic prostate cancer in the bones. Research looking into the ability of fDM to assess bone cancer response is remarkable given that currently there are no ways to measure response in the bone. “We are only on the first or second patient in terms of looking at the bone. It is far too early to make claims about the bone,” said Dr. Hamstra. “We have promising data in the animal models for bone. That has suggested that diffusion MR may be useful [in humans].”
This unique method of analysis was licensed to Cedara Software (Cedara), a Merge Healthcare Company, developer of medical imaging software and custom-engineered solutions for the global OEM market. Cedara has commercialized the technology in its I-Response application. In July 2007, the application was released by Varian Medical Systems, who holds sole rights to distribute the tool to the radiation oncology and medical oncology community. The product has received FDA 510(k) approval, the Canadian Medical Device License and has received the European CE Mark as well.
“I-Response will prove to be an invaluable tool in both the medical oncologists and the radiation oncologists arsenal. It evolved, as most tools do, from the realm of academic research; however, in its current commercial form it is ready for use in both medical oncology and radiation oncology as one the first and most promising tools in the arena of tumor response assessment,” said Jon Hollon, product manager for I-Response at Varian Medical Systems.
In addition to fDM analysis, I-Response also includes lesion and PET analysis tools for evaluation. According to Chris Weidmann, product manager oncology for Cedara Software, “No cancer and no treatment have a silver bullet for assessment, and that’s why we look to provide more than one type of analysis so that clinicians can choose based on the patient and treatment.”
Not only does I-Response provide an invaluable tool for optimum patient care, the tool itself offers features, which improve workflow and increase efficiency by enabling clinicians to perform a variety of assessments with results automatically generated. Weidmann noted, “We provide viewing tools, the ability to register data at multiple time points, segmentation tools for contouring, and those are all auto-generated values. We generate all RECIST measurements, WHO measurements and the volume measurements automatically. We support all of that information into a back end data system. Part of the value of the tool is having all of those components in one system.” In addition, fDM can be easily integrated into existing workstations and is capable of being performed on any MR scanner without the use of a tracer, which could lead to faster and more widespread adoption.
Looking towards the future, fDM shows a lot of promise in terms of contributing to a more personalized and adaptive approach to cancer treatment, which could lead to a change in prognosis.
“The arena of early tumor response assessment is just beginning to evolve,” said Hollon. “Varian is excited to be able to offer one of the first and most versatile tools in this arena. We are confident that I-Response will be key in identifying early response trends and therefore will serve as an incredibly valuable tool
in aiding the physician in choosing the most effective form of treatment at the earliest possible stage of treatment.”


 February 13, 2026
February 13, 2026 









